HMGB1 is an endogenous immune adjuvant released by necrotic cells
- PMID: 15272298
- PMCID: PMC1299116
- DOI: 10.1038/sj.embor.7400205
HMGB1 is an endogenous immune adjuvant released by necrotic cells
Abstract
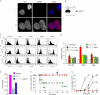
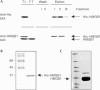
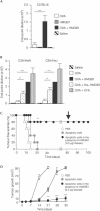
Immune responses against pathogens require that microbial components promote the activation of antigen-presenting cells (APCs). Autoimmune diseases and graft rejections occur in the absence of pathogens; in these conditions, endogenous molecules, the so-called 'innate adjuvants', activate APCs. Necrotic cells contain and release innate adjuvants; necrotic cells also release high-mobility group B1 protein (HMGB1), an abundant and conserved constituent of vertebrate nuclei. Here, we show that necrotic HMGB1(-/-) cells have a reduced ability to activate APCs, and HMGB1 blockade reduces the activation induced by necrotic wild-type cell supernatants. In vivo, HMGB1 enhances the primary antibody responses to soluble antigens and transforms poorly immunogenic apoptotic lymphoma cells into efficient vaccines.
Figures

Similar articles
-
TLR4-dependent activation of dendritic cells by an HMGB1-derived peptide adjuvant.J Transl Med. 2014 Aug 14;12:211. doi: 10.1186/1479-5876-12-211. J Transl Med. 2014. PMID: 25123824 Free PMC article.
-
Generation of the Fluorescent HMGB1-GFP Fusion Protein in Insect Cells and Evaluation of its Immunogenicity in Two Mice Models.Protein Pept Lett. 2017;24(6):545-550. doi: 10.2174/0929866524666170404161649. Protein Pept Lett. 2017. PMID: 28393684
-
CoVaccine HT™ adjuvant is superior to Freund's adjuvants in eliciting antibodies against the endogenous alarmin HMGB1.J Immunol Methods. 2016 Dec;439:37-43. doi: 10.1016/j.jim.2016.09.008. Epub 2016 Sep 30. J Immunol Methods. 2016. PMID: 27693642
-
Sensing necrotic cells.Adv Exp Med Biol. 2012;738:144-52. doi: 10.1007/978-1-4614-1680-7_9. Adv Exp Med Biol. 2012. PMID: 22399378 Review.
-
High-mobility group box 1 (HMGB1) protein: friend and foe.Cytokine Growth Factor Rev. 2006 Jun;17(3):189-201. doi: 10.1016/j.cytogfr.2006.01.003. Epub 2006 Mar 2. Cytokine Growth Factor Rev. 2006. PMID: 16513409 Review.
Cited by
-
CXCR4 engagement triggers CD47 internalization and antitumor immunization in a mouse model of mesothelioma.EMBO Mol Med. 2021 Jun 7;13(6):e12344. doi: 10.15252/emmm.202012344. Epub 2021 May 6. EMBO Mol Med. 2021. PMID: 33956406 Free PMC article.
-
Molecular Behavior of HMGB1 in the Cochlea Following Noise Exposure and in vitro.Front Cell Dev Biol. 2021 Feb 25;9:642946. doi: 10.3389/fcell.2021.642946. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33732708 Free PMC article.
-
Data-Driven Mathematical Model of Osteosarcoma.Cancers (Basel). 2021 May 14;13(10):2367. doi: 10.3390/cancers13102367. Cancers (Basel). 2021. PMID: 34068946 Free PMC article.
-
Unconventional secretion is a major contributor of cancer cell line secretomes.Mol Cell Proteomics. 2013 May;12(5):1046-60. doi: 10.1074/mcp.M112.021618. Epub 2012 Dec 26. Mol Cell Proteomics. 2013. PMID: 23268930 Free PMC article.
-
Role of Alarmins in the Pathogenesis of Systemic Sclerosis.Int J Mol Sci. 2020 Jul 15;21(14):4985. doi: 10.3390/ijms21144985. Int J Mol Sci. 2020. PMID: 32679721 Free PMC article. Review.
References
-
- Andersson U, Erlandsson-Harris H (2004) HMGB1 is a potent trigger of arthritis. J Intern Med 255: 344–350 - PubMed
-
- Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ (2002) HMGB1 as a DNA-binding cytokine. J Leukoc Biol 72: 1084–1091 - PubMed
-
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol 12: 1539–1546 - PubMed
-
- Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA (1997) Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol 159: 5391–5399 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

