Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network
- PMID: 15269279
- PMCID: PMC519138
- DOI: 10.1091/mbc.e03-12-0872
Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network
Abstract
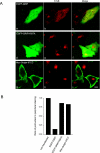
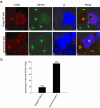
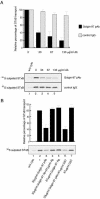
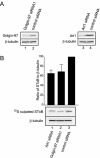
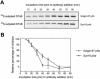
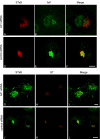
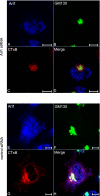
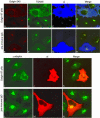
The precise cellular function of Arl1 and its effectors, the GRIP domain Golgins, is not resolved, despite our recent understanding that Arl1 regulates the membrane recruitment of these Golgins. In this report, we describe our functional study of Golgin-97. Using a Shiga toxin B fragment (STxB)-based in vitro transport assay, we demonstrated that Golgin-97 plays a role in transport from the endosome to the trans-Golgi network (TGN). The recombinant GRIP domain of Golgin-97 as well as antibodies against Golgin-97 inhibited the transport of STxB in vitro. Membrane-associated Golgin-97, but not its cytosolic pool, was required in the in vitro transport assay. The kinetic characterization of inhibition by anti-Golgin-97 antibody in comparison with anti-Syntaxin 16 antibody established that Golgin-97 acts before Syntaxin 16 in endosome-to-TGN transport. Knock down of Golgin-97 or Arl1 by their respective small interference RNAs (siRNAs) also significantly inhibited the transport of STxB to the Golgi in vivo. In siRNA-treated cells with reduced levels of Arl1, internalized STxB was instead distributed peripherally. Microinjection of Golgin-97 antibody led to the fragmentation of Golgi apparatus and the arrested transport to the Golgi of internalized Cholera toxin B fragment. We suggest that Golgin-97 may function as a tethering molecule in endosome-to-TGN retrograde traffic.
Figures



Similar articles
-
Functional analysis of Arl1 and golgin-97 in endosome-to-TGN transport using recombinant Shiga toxin B fragment.Methods Enzymol. 2005;404:442-53. doi: 10.1016/S0076-6879(05)04039-5. Methods Enzymol. 2005. PMID: 16413290
-
Arfaptin-1 negatively regulates Arl1-mediated retrograde transport.PLoS One. 2015 Mar 19;10(3):e0118743. doi: 10.1371/journal.pone.0118743. eCollection 2015. PLoS One. 2015. PMID: 25789876 Free PMC article.
-
The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure.Traffic. 2007 Jun;8(6):758-73. doi: 10.1111/j.1600-0854.2007.00563.x. Epub 2007 May 4. Traffic. 2007. PMID: 17488291
-
Multiple activities of Arl1 GTPase in the trans-Golgi network.J Cell Sci. 2017 May 15;130(10):1691-1699. doi: 10.1242/jcs.201319. Epub 2017 May 3. J Cell Sci. 2017. PMID: 28468990 Review.
-
Domains of the TGN: coats, tethers and G proteins.Traffic. 2004 May;5(5):315-26. doi: 10.1111/j.1398-9219.2004.00182.x. Traffic. 2004. PMID: 15086781 Review.
Cited by
-
A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling.Mol Biol Cell. 2006 Oct;17(10):4353-63. doi: 10.1091/mbc.e06-02-0153. Epub 2006 Aug 2. Mol Biol Cell. 2006. PMID: 16885419 Free PMC article.
-
Golgin-97 Targets Ectopically Expressed Inward Rectifying Potassium Channel, Kir2.1, to the trans-Golgi Network in COS-7 Cells.Front Physiol. 2018 Aug 3;9:1070. doi: 10.3389/fphys.2018.01070. eCollection 2018. Front Physiol. 2018. PMID: 30123141 Free PMC article.
-
FIP1/RCP binding to Golgin-97 regulates retrograde transport from recycling endosomes to the trans-Golgi network.Mol Biol Cell. 2010 Sep 1;21(17):3041-53. doi: 10.1091/mbc.E10-04-0313. Epub 2010 Jul 7. Mol Biol Cell. 2010. PMID: 20610657 Free PMC article.
-
Hypoxia-regulated carbonic anhydrase IX (CAIX) protein is an independent prognostic indicator in triple negative breast cancer.Breast Cancer Res. 2022 Jun 3;24(1):38. doi: 10.1186/s13058-022-01532-0. Breast Cancer Res. 2022. PMID: 35659359 Free PMC article.
-
Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum.Toxins (Basel). 2010 Mar;2(3):310-25. doi: 10.3390/toxins2030310. Epub 2010 Mar 5. Toxins (Basel). 2010. PMID: 22069586 Free PMC article. Review.
References
-
- Allan, B.B., Moyer, B.D., and Balch, W.E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444-448. - PubMed
-
- Antonny, B., and Schekman, R. (2001). ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol. 13, 438-443. - PubMed
-
- Barr, F.A. (1999). A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr. Biol. 9, 381-384. - PubMed
-
- Barr, F.A., Puype, M., Vandekerckhove, J., and Warren, G. (1997). GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91, 253-262. - PubMed
-
- Barr, F.A., and Short, B. (2003). Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15, 405-413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

