Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments
- PMID: 15263019
- PMCID: PMC2172308
- DOI: 10.1083/jcb.200401078
Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments
Abstract
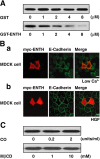
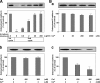
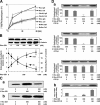
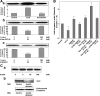
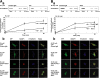
E-cadherin is a key cell-cell adhesion molecule at adherens junctions (AJs) and undergoes endocytosis when AJs are disrupted by the action of extracellular signals. To elucidate the mechanism of this endocytosis, we developed here a new cell-free assay system for this reaction using the AJ-enriched fraction from rat liver. We found here that non-trans-interacting, but not trans-interacting, E-cadherin underwent endocytosis in a clathrin-dependent manner. The endocytosis of trans-interacting E-cadherin was inhibited by Rac and Cdc42 small G proteins, which were activated by trans-interacting E-cadherin or trans-interacting nectins, which are known to induce the formation of AJs in cooperation with E-cadherin. This inhibition was mediated by reorganization of the actin cytoskeleton by Rac and Cdc42 through IQGAP1, an actin filament-binding protein and a downstream target of Rac and Cdc42. These results indicate the important role of the Rac/Cdc42-IQGAP1 system in the dynamic organization and maintenance of the E-cadherin-based AJs.
Copyright The Rockerfeller University Press
Figures


Similar articles
-
A cell-free assay for endocytosis of E-cadherin.Methods Mol Biol. 2008;440:77-87. doi: 10.1007/978-1-59745-178-9_6. Methods Mol Biol. 2008. PMID: 18369938
-
Interactions among IQGAP1, Cdc42, and the cadherin/catenin protein complex regulate Sertoli-germ cell adherens junction dynamics in the testis.J Cell Physiol. 2005 Jan;202(1):49-66. doi: 10.1002/jcp.20098. J Cell Physiol. 2005. PMID: 15389538
-
Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion.Biochem Biophys Res Commun. 1999 Aug 19;262(1):1-6. doi: 10.1006/bbrc.1999.1122. Biochem Biophys Res Commun. 1999. PMID: 10448058 Review.
-
Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway.Oncogene. 2006 Jan 5;25(1):8-19. doi: 10.1038/sj.onc.1209010. Oncogene. 2006. PMID: 16170364
-
[Roles of IQGAP1 in E-cadherin-mediated cell-cell adhesion].Tanpakushitsu Kakusan Koso. 2006 May;51(6 Suppl):648-53. Tanpakushitsu Kakusan Koso. 2006. PMID: 16719325 Review. Japanese. No abstract available.
Cited by
-
Signaling from the podocyte intercellular junction to the actin cytoskeleton.Semin Nephrol. 2012 Jul;32(4):307-18. doi: 10.1016/j.semnephrol.2012.06.002. Semin Nephrol. 2012. PMID: 22958485 Free PMC article. Review.
-
Discovering the molecular components of intercellular junctions--a historical view.Cold Spring Harb Perspect Biol. 2009 Sep;1(3):a003061. doi: 10.1101/cshperspect.a003061. Cold Spring Harb Perspect Biol. 2009. PMID: 20066111 Free PMC article. Review.
-
Tumour follower cells: A novel driver of leader cells in collective invasion (Review).Int J Oncol. 2023 Oct;63(4):115. doi: 10.3892/ijo.2023.5563. Epub 2023 Aug 24. Int J Oncol. 2023. PMID: 37615176 Free PMC article. Review.
-
Rac1 regulates pancreatic islet morphogenesis.BMC Dev Biol. 2009 Jan 6;9:2. doi: 10.1186/1471-213X-9-2. BMC Dev Biol. 2009. PMID: 19126201 Free PMC article.
-
Inter-dependent apical microtubule and actin dynamics orchestrate centrosome retention and neuronal delamination.Elife. 2017 Oct 23;6:e26215. doi: 10.7554/eLife.26215. Elife. 2017. PMID: 29058679 Free PMC article.
References
-
- Adams, C.L., and W.J. Nelson. 1998. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10:572–577. - PubMed
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2000. The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113:1319–1334. - PubMed
-
- Arribas, J., and A. Borroto. 2002. Protein ectodomain shedding. Chem. Rev. 102:4627–4638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

