What is the best size descriptor to use for pharmacokinetic studies in the obese?
- PMID: 15255794
- PMCID: PMC1884581
- DOI: 10.1111/j.1365-2125.2004.02157.x
What is the best size descriptor to use for pharmacokinetic studies in the obese?
Abstract
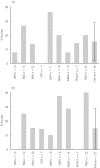
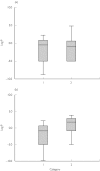
The prevalence of obesity in the western world is dramatically rising, with many of these individuals requiring therapeutic intervention for a variety of disease states. Despite the growing prevalence of obesity there is a paucity of information describing how doses should be adjusted, or indeed whether they need to be adjusted, in the clinical setting. This review is aimed at identifying which descriptors of body size provide the most information about the relationship between dose and concentration in the obese. The size descriptors, weight, lean body weight, ideal body weight, body surface area, body mass index, fat-free mass, percent ideal body weight, adjusted body weight and predicted normal body weight were considered as potential size descriptors. We conducted an extensive review of the literature to identify studies that have assessed the quantitative relationship between the parameters clearance (CL) and volume of distribution (V) and these descriptors of body size. Surprisingly few studies have addressed the relationship between obesity and CL or V in a quantitative manner. Despite the lack of studies there were consistent findings: (i) most studies found total body weight to be the best descriptor of V. A further analysis of the studies that have addressed V found that total body weight or another descriptor that incorporated fat mass was the preferred descriptor for drugs that have high lipophilicity; (ii) in contrast, CL was best described by lean body mass and no apparent relationship between lipophilicity or clearance mechanism and preference for body size descriptor was found. In conclusion, no single descriptor described the influence of body size on both CL and V equally well. For drugs that are dosed chronically, and therefore CL is of primary concern, dosing for obese patients should not be based on their total weight. If a weight-based dose individualization is required then we would suggest that chronic drug dosing in the obese subject should be based on lean body weight, at least until a more robust size descriptor becomes available.
Figures
Similar articles
-
Effect of obesity on the pharmacokinetics of drugs in humans.Clin Pharmacokinet. 2010;49(2):71-87. doi: 10.2165/11318100-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20067334 Review.
-
Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese.J Clin Oncol. 2007 Oct 20;25(30):4707-13. doi: 10.1200/JCO.2007.11.2938. J Clin Oncol. 2007. PMID: 17947717
-
The relationship between drug clearance and body size: systematic review and meta-analysis of the literature published from 2000 to 2007.Clin Pharmacokinet. 2012 May 1;51(5):319-30. doi: 10.2165/11598930-000000000-00000. Clin Pharmacokinet. 2012. PMID: 22439649 Review.
-
Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults.Pharmacotherapy. 2012 Sep;32(9):856-68. doi: 10.1002/j.1875-9114.2012.01108.x. Epub 2012 Jun 18. Pharmacotherapy. 2012. PMID: 22711238 Review.
-
Evaluating the Relationship Between Lean Liver Volume and Fat-Free Mass.Clin Pharmacokinet. 2020 Apr;59(4):475-483. doi: 10.1007/s40262-019-00824-7. Clin Pharmacokinet. 2020. PMID: 31583612
Cited by
-
A Review of the Toxicologic Implications of Obesity.J Med Toxicol. 2015 Sep;11(3):342-54. doi: 10.1007/s13181-015-0488-6. J Med Toxicol. 2015. PMID: 26108709 Free PMC article. Review.
-
Pharmacokinetics and drug dosing in obese children.J Pediatr Pharmacol Ther. 2010 Apr;15(2):94-109. J Pediatr Pharmacol Ther. 2010. PMID: 22477800 Free PMC article.
-
Population pharmacokinetics and pharmacodynamics of hydroxyurea in sickle cell anemia patients, a basis for optimizing the dosing regimen.Orphanet J Rare Dis. 2011 May 28;6:30. doi: 10.1186/1750-1172-6-30. Orphanet J Rare Dis. 2011. PMID: 21619673 Free PMC article. Clinical Trial.
-
A Review of the Methods and Associated Mathematical Models Used in the Measurement of Fat-Free Mass.Clin Pharmacokinet. 2018 Jul;57(7):781-795. doi: 10.1007/s40262-017-0622-5. Clin Pharmacokinet. 2018. PMID: 29330781 Review.
-
Monitoring of gentamicin serum concentrations in obstetrics and gynaecology patients in Namibia.Int J Clin Pharm. 2018 Jun;40(3):520-525. doi: 10.1007/s11096-018-0626-8. Epub 2018 Mar 28. Int J Clin Pharm. 2018. PMID: 29594677
References
-
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. - PubMed
-
- National Institute of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: the Evidence Report. Bethesda, MD: US Department of Health and Human Services; 1998. - PubMed
-
- Anderson JW, Konz EC. Obesity and disease management: effects of weight loss on comorbid conditions. Obes Res. 2001;9:326S–334S. - PubMed
-
- World Health Organization Report of a WHO Consultation on Obesity: Preventing and Managing the Global Epidemic. Geneva: WHO; 1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

