Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes
- PMID: 15254595
- PMCID: PMC449750
- DOI: 10.1172/JCI21583
Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes
Erratum in
- J Clin Invest. 2004 Aug;114(4):599
Abstract
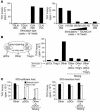
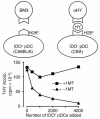
One mechanism contributing to immunologic unresponsiveness toward tumors may be presentation of tumor antigens by tolerogenic host APCs. We show that mouse tumor-draining LNs (TDLNs) contained a subset of plasmacytoid DCs (pDCs) that constitutively expressed immunosuppressive levels of the enzyme indoleamine 2,3-dioxygenase (IDO). Despite comprising only 0.5% of LN cells, these pDCs in vitro potently suppressed T cell responses to antigens presented by the pDCs themselves and also, in a dominant fashion, suppressed T cell responses to third-party antigens presented by nonsuppressive APCs. Adoptive transfer of DCs from TDLNs into naive hosts created profound local T cell anergy, specifically toward antigens expressed by the transferred DCs. Anergy was prevented by targeted disruption of the IDO gene in the DCs or by administration of the IDO inhibitor drug 1-methyl-D-tryptophan to recipient mice. Within the population of pDCs, the majority of the functional IDO-mediated suppressor activity segregated with a novel subset of pDCs coexpressing the B-lineage marker CD19. We hypothesize that IDO-mediated suppression by pDCs in TDLNs creates a local microenvironment that is potently suppressive of host antitumor T cell responses.
Figures


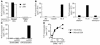
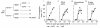

Similar articles
-
Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase.J Clin Invest. 2007 Sep;117(9):2570-82. doi: 10.1172/JCI31911. J Clin Invest. 2007. PMID: 17710230 Free PMC article.
-
GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase.Immunity. 2005 May;22(5):633-42. doi: 10.1016/j.immuni.2005.03.013. Immunity. 2005. PMID: 15894280
-
Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes.Blood. 2009 Jun 11;113(24):6102-11. doi: 10.1182/blood-2008-12-195354. Epub 2009 Apr 14. Blood. 2009. PMID: 19366986 Free PMC article.
-
T cell regulatory plasmacytoid dendritic cells expressing indoleamine 2,3 dioxygenase.Handb Exp Pharmacol. 2009;(188):165-96. doi: 10.1007/978-3-540-71029-5_8. Handb Exp Pharmacol. 2009. PMID: 19031026 Free PMC article. Review.
-
Indoleamine 2,3-dioxygenase: from catalyst to signaling function.Eur J Immunol. 2012 Aug;42(8):1932-7. doi: 10.1002/eji.201242572. Eur J Immunol. 2012. PMID: 22865044 Review.
Cited by
-
The role of indoleamine 2, 3 dioxygenase in regulating host immunity to leishmania infection.J Biomed Sci. 2012 Jan 9;19(1):5. doi: 10.1186/1423-0127-19-5. J Biomed Sci. 2012. PMID: 22230608 Free PMC article.
-
Immune cell profile of sentinel lymph nodes in patients with malignant melanoma - FOXP3+ cell density in cases with positive sentinel node status is associated with unfavorable clinical outcome.J Transl Med. 2013 Feb 18;11:43. doi: 10.1186/1479-5876-11-43. J Transl Med. 2013. PMID: 23418928 Free PMC article.
-
Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4.J Exp Med. 2013 Jul 1;210(7):1389-402. doi: 10.1084/jem.20130066. Epub 2013 Jun 10. J Exp Med. 2013. PMID: 23752227 Free PMC article.
-
Effects of indoleamine 2,3-dioxygenase inhibitor in non-Hodgkin lymphoma model mice.Int J Hematol. 2015 Sep;102(3):327-34. doi: 10.1007/s12185-015-1835-8. Epub 2015 Aug 5. Int J Hematol. 2015. PMID: 26243621
-
Tumor associated regulatory dendritic cells.Semin Cancer Biol. 2012 Aug;22(4):298-306. doi: 10.1016/j.semcancer.2012.02.010. Epub 2012 Mar 6. Semin Cancer Biol. 2012. PMID: 22414911 Free PMC article. Review.
References
-
- Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001;2:293–299. - PubMed
-
- Ochsenbein AF, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. - PubMed
-
- Spiotto MT, et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

