Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase
- PMID: 15239838
- PMCID: PMC471571
- DOI: 10.1186/1477-7827-2-54
Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase
Abstract
Background: In the endometrium the steroid hormone progesterone (P), acting through its nuclear receptors, regulates the expression of specific target genes and gene networks required for endometrial maturation. Proper endometrial maturation is considered a requirement for embryo implantation. Endometrial receptivity is a complex process that is spatially and temporally restricted and the identity of genes that regulate receptivity has been pursued by a number of investigators.
Methods: In this study we have used high density oligonucleotide microarrays to screen for changes in mRNA transcript levels between normal proliferative and adequate secretory phases in Rhesus monkey artificial menstrual cycles. Biotinylated cRNA was prepared from day 13 and days 21-23 of the reproductive cycle and transcript levels were compared by hybridization to Affymetrix HG-U95A arrays.
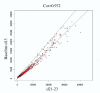
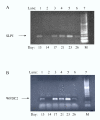
Results: Of approximately 12,000 genes profiled, we identified 108 genes that were significantly regulated during the shift from a proliferative to an adequate secretory endometrium. Of these genes, 39 were up-regulated at days 21-23 versus day 13, and 69 were down-regulated. Genes up-regulated in P-dominant tissue included: secretoglobin (uteroglobin), histone 2A, polo-like kinase (PLK), spermidine/spermine acetyltransferase 2 (SAT2), secretory leukocyte protease inhibitor (SLPI) and metallothionein 1G (MT1G), all of which have been previously documented as elevated in the Rhesus monkey or human endometrium during the secretory phase. Genes down-regulated included: transforming growth factor beta-induced (TGFBI or BIGH3), matrix metalloproteinase 11 (stromelysin 3), proenkephalin (PENK), cysteine/glycine-rich protein 2 (CSRP2), collagen type VII alpha 1 (COL7A1), secreted frizzled-related protein 4 (SFRP4), progesterone receptor membrane component 1 (PGRMC1), chemokine (C-X-C) ligand 12 (CXCL12) and biglycan (BGN). In addition, many novel/unknown genes were also identified. Validation of array data was performed by semi-quantitative RT-PCR of two selected up-regulated genes using temporal (cycle day specific) endometrial cDNA populations. This approach confirmed up-regulation of WAP four-disulfide core domain 2 (WFDC2) and SLPI during the expected window of receptivity.
Conclusion: The identification of P-regulated genes and gene pathways in the primate endometrium is expected to be an important first step in elucidating the cellular processes necessary for the development of a receptive environment for implantation.
Figures
Similar articles
-
Temporal regulation of gene expression during the expected window of receptivity in the rhesus monkey endometrium.Biol Reprod. 2003 Nov;69(5):1593-9. doi: 10.1095/biolreprod.103.017525. Epub 2003 Jul 9. Biol Reprod. 2003. PMID: 12855598
-
Analysis of differential gene regulation in adequate versus inadequate secretory-phase endometrial complementary deoxyribonucleic acid populations from the rhesus monkey.Endocrinology. 1996 Nov;137(11):4844-50. doi: 10.1210/endo.137.11.8895355. Endocrinology. 1996. PMID: 8895355
-
Global gene profiling in human endometrium during the window of implantation.Endocrinology. 2002 Jun;143(6):2119-38. doi: 10.1210/endo.143.6.8885. Endocrinology. 2002. PMID: 12021176
-
Progesterone-regulated gene expression in the primate endometrium.Semin Reprod Endocrinol. 1999;17(3):241-55. doi: 10.1055/s-2007-1016232. Semin Reprod Endocrinol. 1999. PMID: 10797943 Review.
-
Gene expression in the rhesus monkey endometrium: differential display and laser capture microdissection.Front Biosci. 2003 May 1;8:d551-8. doi: 10.2741/1035. Front Biosci. 2003. PMID: 12700029 Review.
Cited by
-
Expression of nuclear progesterone receptor and progesterone receptor membrane components 1 and 2 in the oviduct of cyclic and pregnant cows during the post-ovulation period.Reprod Biol Endocrinol. 2012 Sep 7;10:76. doi: 10.1186/1477-7827-10-76. Reprod Biol Endocrinol. 2012. PMID: 22958265 Free PMC article.
-
Cell Models for the Study of Sex Steroid Hormone Neurobiology.J Steroids Horm Sci. 2012;S2:003. doi: 10.4172/2157-7536.s2-003. J Steroids Horm Sci. 2012. PMID: 22860237 Free PMC article.
-
Delineating the prime mover action of progesterone for endometrial receptivity in primates.Indian J Med Res. 2014 Nov;140 Suppl(Suppl 1):S130-6. Indian J Med Res. 2014. PMID: 25673534 Free PMC article. Review.
-
Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes.PLoS One. 2012;7(4):e35859. doi: 10.1371/journal.pone.0035859. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545144 Free PMC article.
-
Comparison in gene expression of secretory human endometrium using laser microdissection.Reprod Biol Endocrinol. 2004 Sep 17;2:66. doi: 10.1186/1477-7827-2-66. Reprod Biol Endocrinol. 2004. PMID: 15373944 Free PMC article.
References
-
- Bartelmez GW, Corner GW, Hartman CG. Cyclic changes in the endometrium of the rhesus monkey (Macaca mulatta). Contrib Embryol. 1951;34:99–144.
-
- Ferenczy A, Bergeron C. Histology of the human endometrium: From birth to senescence. Ann NY Acad Sci. 1991;622:6–27. - PubMed
-
- Gurpide E, Tseng L. Induction of human endometrial oestradiol dehydrogenase by progestins. Endocrinology. 1975;97:825–833. - PubMed
-
- Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990;11:266–301. - PubMed
-
- Fay TN, Grudzinskas JG. Human endometrial peptides: A review of their potential role in implantation and placentation. Hum Reprod. 1991;6:1311–1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

