Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3
- PMID: 15232609
- PMCID: PMC437966
- DOI: 10.1172/JCI20509
Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3
Abstract
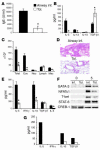
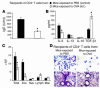
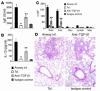
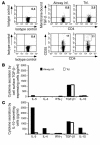
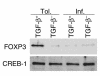
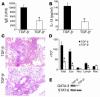
Under normal circumstances, the respiratory tract maintains immune tolerance in the face of constant antigen provocation. Using a murine model of tolerance induced by repeated exposure to a low dose of aerosolized antigen, we show an important contribution by CD4(+) T cells in the establishment and maintenance of tolerance. The CD4(+) T cells expressed both cell surface and soluble TGF-beta and inhibited the development of an allergic phenotype when adoptively transferred to naive recipient mice. While cells expressing cell surface TGF-beta were detectable in mice with inflammation, albeit at a lower frequency compared with that in tolerized mice, only those from tolerized mice expressed FOXP3. Blockade of TGF-beta in vitro and in vivo interfered with immunosuppression. Although cells that expressed TGF-beta on the cell surface (TGF-beta(+)), as well as the ones that did not (TGF-beta(-)), secreted equivalent levels of soluble TGF-beta, only the former were able to blunt the development of an allergic phenotype in mice. Strikingly, separation of the TGF-beta(+) cells from the rest of the cells allowed the TGF-beta(-) cells to proliferate in response to antigen. We propose a model of antigen-induced tolerance that involves cell-cell contact with regulatory CD4(+) T cells that coexpress membrane-bound TGF-beta and FOXP3.
Figures

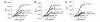


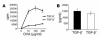
Similar articles
-
Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3.J Exp Med. 2003 Dec 15;198(12):1875-86. doi: 10.1084/jem.20030152. J Exp Med. 2003. PMID: 14676299 Free PMC article.
-
Involvement of Foxp3-expressing CD4+ CD25+ regulatory T cells in the development of tolerance induced by transforming growth factor-beta2-treated antigen-presenting cells.Immunology. 2008 Jul;124(3):304-14. doi: 10.1111/j.1365-2567.2007.02769.x. Epub 2008 Feb 11. Immunology. 2008. PMID: 18266851 Free PMC article.
-
Complementary role of CD4+CD25+ regulatory T cells and TGF-beta in oral tolerance.J Leukoc Biol. 2005 Jun;77(6):906-13. doi: 10.1189/jlb.1004599. Epub 2005 Mar 9. J Leukoc Biol. 2005. PMID: 15758078
-
Tolerogenic dendritic cells induced the enrichment of CD4+Foxp3+ regulatory T cells via TGF-β in mesenteric lymph nodes of murine LPS-induced tolerance model.Clin Immunol. 2018 Dec;197:118-129. doi: 10.1016/j.clim.2018.09.010. Epub 2018 Sep 21. Clin Immunol. 2018. PMID: 30248398
-
Transforming growth factor-beta-induced regulatory T cells referee inflammatory and autoimmune diseases.Arthritis Res Ther. 2005;7(2):62-8. doi: 10.1186/ar1504. Epub 2005 Jan 24. Arthritis Res Ther. 2005. PMID: 15743491 Free PMC article. Review.
Cited by
-
Therapeutical measures to control airway tolerance in asthma and lung cancer.Front Immunol. 2012 Jul 26;3:216. doi: 10.3389/fimmu.2012.00216. eCollection 2012. Front Immunol. 2012. PMID: 22855687 Free PMC article.
-
The rabbit as a model for studying lung disease and stem cell therapy.Biomed Res Int. 2013;2013:691830. doi: 10.1155/2013/691830. Epub 2013 Apr 8. Biomed Res Int. 2013. PMID: 23653896 Free PMC article. Review.
-
Cutting Edge: Dual Function of PPARγ in CD11c+ Cells Ensures Immune Tolerance in the Airways.J Immunol. 2015 Jul 15;195(2):431-5. doi: 10.4049/jimmunol.1500474. Epub 2015 Jun 10. J Immunol. 2015. PMID: 26062999 Free PMC article.
-
Regulatory T Cells, a Potent Immunoregulatory Target for CAM Researchers: Modulating Allergic and Infectious Disease Pathology (II).Evid Based Complement Alternat Med. 2006 Jun;3(2):209-15. doi: 10.1093/ecam/nel020. Epub 2006 Apr 19. Evid Based Complement Alternat Med. 2006. PMID: 16786050 Free PMC article.
-
Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes.J Exp Med. 2005 Apr 18;201(8):1333-46. doi: 10.1084/jem.20042398. J Exp Med. 2005. PMID: 15837817 Free PMC article.
References
-
- Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 2001;1:69–75. - PubMed
-
- Braun-Fahrlander C, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002;347:869–877. - PubMed
-
- McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869–1871. - PubMed
-
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2001;2:725–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

