The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1
- PMID: 15226448
- PMCID: PMC434242
- DOI: 10.1128/MCB.24.14.6488-6500.2004
The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1
Abstract
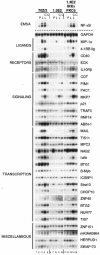
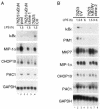
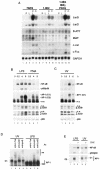
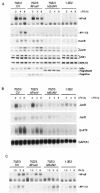
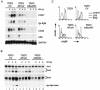
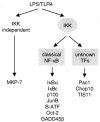
Toll-like receptors (TLRs) recognize conserved products of microbial pathogens to initiate the innate immune response. TLR4 signaling is triggered upon binding of lipopolysaccharides (LPS) from gram-negative bacteria. Using comparative gene expression profiling, we demonstrate a master regulatory role of IkappaB kinase (IKK)/NF-kappaB signaling for immediate-early gene induction after LPS engagement in precursor B cells. IKK/NF-kappaB signaling controls a large panel of gene products associated with signaling and transcriptional activation and repression. Intriguingly, the induction of AP-1 activity by LPS in precursor B cells and primary dendritic cells fully depends on the IKK/NF-kappaB pathway, which promotes expression of several AP-1 family members, including JunB, JunD, and B-ATF. In pre-B cells, AP-1 augments induction of a subset of primary NF-kappaB targets, as shown for chemokine receptor 7 (CCR7) and immunoglobulin kappa light chain. Thus, our data illustrate that NF-kappaB orchestrates immediate-early effects of LPS signaling and controls secondary AP-1 activation to mount an appropriate biological response.
Figures

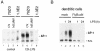
Similar articles
-
Ras and mitogen-activated protein kinase kinase kinase-1 coregulate activator protein-1- and nuclear factor-kappaB-mediated gene expression in airway epithelial cells.Am J Respir Cell Mol Biol. 2003 Jun;28(6):762-9. doi: 10.1165/rcmb.2002-0261OC. Epub 2003 Jan 31. Am J Respir Cell Mol Biol. 2003. PMID: 12600818
-
Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation.Oncogene. 2003 Jan 23;22(3):412-25. doi: 10.1038/sj.onc.1206132. Oncogene. 2003. PMID: 12545162
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity.Mol Cell Biol. 2004 Sep;24(17):7806-19. doi: 10.1128/MCB.24.17.7806-7819.2004. Mol Cell Biol. 2004. PMID: 15314185 Free PMC article.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection.Int J Mol Sci. 2019 Apr 18;20(8):1912. doi: 10.3390/ijms20081912. Int J Mol Sci. 2019. PMID: 31003422 Free PMC article. Review.
-
Hydrogen peroxide modulates immunoglobulin expression by targeting the 3'Igh regulatory region through an NFκB-dependent mechanism.Free Radic Res. 2011 Jul;45(7):796-809. doi: 10.3109/10715762.2011.581280. Epub 2011 May 20. Free Radic Res. 2011. PMID: 21599461 Free PMC article.
-
AIP augments CARMA1-BCL10-MALT1 complex formation to facilitate NF-κB signaling upon T cell activation.Cell Commun Signal. 2014 Jul 22;12:49. doi: 10.1186/s12964-014-0049-7. Cell Commun Signal. 2014. PMID: 25245034 Free PMC article.
-
Molecular stripping in the NF-κB/IκB/DNA genetic regulatory network.Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):110-5. doi: 10.1073/pnas.1520483112. Epub 2015 Dec 23. Proc Natl Acad Sci U S A. 2016. PMID: 26699500 Free PMC article.
-
Modulation of Heme-Induced Inflammation Using MicroRNA-Loaded Liposomes: Implications for Hemolytic Disorders Such as Malaria and Sickle Cell Disease.Int J Mol Sci. 2023 Nov 29;24(23):16934. doi: 10.3390/ijms242316934. Int J Mol Sci. 2023. PMID: 38069257 Free PMC article.
References
-
- Alkema, M. J., J. Jacobs, J. W. Voncken, N. A. Jenkins, N. G. Copeland, D. P. Satijn, A. P. Otte, A. Berns, and M. van Lohuizen. 1997. MPc2, a new murine homolog of the Drosophila polycomb protein is a member of the mouse polycomb transcriptional repressor complex. J. Mol. Biol. 273:993-1003. - PubMed
-
- Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. - PubMed
-
- Bendall, H. H., D. C. Scherer, C. R. Edson, D. W. Ballard, and E. M. Oltz. 1997. Transcription factor NF-kappa B regulates inducible Oct-2 gene expression in precursor B lymphocytes J. Biol. Chem. 272:28826-28828. - PubMed
-
- Briskin, M., M. D. Kuwabara, D. S. Sigman, and R. Wall. 1988. Induction of kappa transcription by interferon-gamma without activation of NF-kappa B. Science 242:1036-1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
