Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway
- PMID: 15226445
- PMCID: PMC434240
- DOI: 10.1128/MCB.24.14.6456-6466.2004
Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway
Abstract
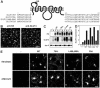
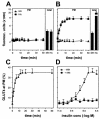
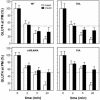
The insulin-responsive glucose transporter GLUT4 plays an essential role in glucose homeostasis. A novel assay was used to study GLUT4 trafficking in 3T3-L1 fibroblasts/preadipocytes and adipocytes. Whereas insulin stimulated GLUT4 translocation to the plasma membrane in both cell types, in nonstimulated fibroblasts GLUT4 readily cycled between endosomes and the plasma membrane, while this was not the case in adipocytes. This efficient retention in basal adipocytes was mediated in part by a C-terminal targeting motif in GLUT4. Insulin caused a sevenfold increase in the amount of GLUT4 molecules present in a trafficking cycle that included the plasma membrane. Strikingly, the magnitude of this increase correlated with the insulin dose, indicating that the insulin-induced appearance of GLUT4 at the plasma membrane cannot be explained solely by a kinetic change in the recycling of a fixed intracellular GLUT4 pool. These data are consistent with a model in which GLUT4 is present in a storage compartment, from where it is released in a graded or quantal manner upon insulin stimulation and in which released GLUT4 continuously cycles between intracellular compartments and the cell surface independently of the nonreleased pool.
Figures




Similar articles
-
Insulin stimulates the entry of GLUT4 into the endosomal recycling pathway by a quantal mechanism.Traffic. 2004 Oct;5(10):763-71. doi: 10.1111/j.1600-0854.2004.00218.x. Traffic. 2004. PMID: 15355512
-
Quantification of SNARE protein levels in 3T3-L1 adipocytes: implications for insulin-stimulated glucose transport.Biochem Biophys Res Commun. 2000 Apr 21;270(3):841-5. doi: 10.1006/bbrc.2000.2525. Biochem Biophys Res Commun. 2000. PMID: 10772913
-
Role for the microtubule cytoskeleton in GLUT4 vesicle trafficking and in the regulation of insulin-stimulated glucose uptake.Biochem J. 2000 Dec 1;352 Pt 2(Pt 2):267-76. Biochem J. 2000. PMID: 11085918 Free PMC article.
-
Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes.Endocr Rev. 2004 Apr;25(2):177-204. doi: 10.1210/er.2003-0011. Endocr Rev. 2004. PMID: 15082519 Review.
-
Targeting motifs in GLUT4 (review).Mol Membr Biol. 2001 Oct-Dec;18(4):257-64. doi: 10.1080/09687680110090780. Mol Membr Biol. 2001. PMID: 11780754 Review.
Cited by
-
The glucose transporter 4-regulating protein TUG is essential for highly insulin-responsive glucose uptake in 3T3-L1 adipocytes.J Biol Chem. 2007 Mar 9;282(10):7710-22. doi: 10.1074/jbc.M610824200. Epub 2007 Jan 3. J Biol Chem. 2007. PMID: 17202135 Free PMC article.
-
Loss of AS160 Akt substrate causes Glut4 protein to accumulate in compartments that are primed for fusion in basal adipocytes.J Biol Chem. 2011 Jul 29;286(30):26287-97. doi: 10.1074/jbc.M111.253880. Epub 2011 May 24. J Biol Chem. 2011. PMID: 21613213 Free PMC article.
-
Characterisation of GLUT4 trafficking in HeLa cells: comparable kinetics and orthologous trafficking mechanisms to 3T3-L1 adipocytes.PeerJ. 2020 Mar 5;8:e8751. doi: 10.7717/peerj.8751. eCollection 2020. PeerJ. 2020. PMID: 32185116 Free PMC article.
-
Leveraging genetic diversity to identify small molecules that reverse mouse skeletal muscle insulin resistance.Elife. 2023 Jul 26;12:RP86961. doi: 10.7554/eLife.86961. Elife. 2023. PMID: 37494090 Free PMC article.
-
Prior exercise in humans redistributes intramuscular GLUT4 and enhances insulin-stimulated sarcolemmal and endosomal GLUT4 translocation.Mol Metab. 2020 Sep;39:100998. doi: 10.1016/j.molmet.2020.100998. Epub 2020 Apr 17. Mol Metab. 2020. PMID: 32305516 Free PMC article.
References
-
- Al-Hasani, H., C. S. Hinck, and S. W. Cushman. 1998. Endocytosis of the glucose transporter GLUT4 is mediated by the GTPase dynamin. J. Biol. Chem. 273:17504-17510. - PubMed
-
- Bogan, J. S., N. Hendon, A. E. McKee, T. S. Tsao, and H. F. Lodish. 2003. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature 425:727-733. - PubMed
-
- Bryant, N. J., R. Govers, and D. E. James. 2002. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 3:267-277. - PubMed
-
- Duncan, R. R., J. Greaves, U. K. Wiegand, I. Matskevich, G. Bodammer, D. K. Apps, M. J. Shipston, and R. H. Chow. 2003. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature 422:176-180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
