Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype
- PMID: 15215289
- PMCID: PMC6729216
- DOI: 10.1523/JNEUROSCI.0330-04.2004
Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype
Abstract
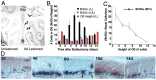
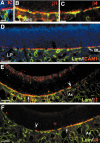
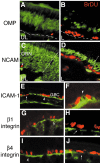
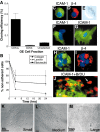
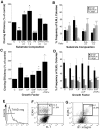
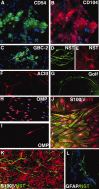
Stem cells of adult regenerative organs share a common goal but few established conserved mechanisms. Within the neural stem cell niche of the mouse olfactory epithelium, we identified a combination of extracellular matrix (ECM) receptors that regulate adhesion and mitosis in non-neural stem cells [intercellular adhesion molecule-1 (ICAM-1), beta1, beta4, and alpha-1, -3, and -6 integrins] and on horizontal basal cells (HBCs), candidate olfactory neuro-epithelial progenitors. Using ECM receptors as our guide, we recreated a defined microenvironment in vitro that mimics olfactory basal lamina and, when supplemented with epidermal growth factor, transforming growth factor alpha, and leukemia inhibitory factor, allows us to preferentially expand multiple clonal adherent colony phenotypes from individual ICAM-1+ and ICAM-1+/beta1 integrin+-selected HBCs. The most highly mitotic colony-forming HBCs demonstrate multipotency, spontaneously generating more ICAM-positive presumptive HBCs, a combination of olfactory neuroglial progenitors, and neurons of olfactory and potentially nonolfactory phenotypes. HBCs thus possess a conserved adhesion receptor expression profile similar to non-neural stem cells, preferential self-replication in an in vitro environment mimicking their in vivo niche, and contain subpopulations of cells that can produce multiple differentiated neuronal and glial progeny from within and beyond the olfactory system in vitro.
Figures



Similar articles
-
Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium.Stem Cells. 2008 May;26(5):1298-306. doi: 10.1634/stemcells.2007-0891. Epub 2008 Feb 28. Stem Cells. 2008. PMID: 18308944 Free PMC article.
-
Activating a Reserve Neural Stem Cell Population In Vitro Enables Engraftment and Multipotency after Transplantation.Stem Cell Reports. 2019 Apr 9;12(4):680-695. doi: 10.1016/j.stemcr.2019.02.014. Epub 2019 Mar 28. Stem Cell Reports. 2019. PMID: 30930245 Free PMC article.
-
Human olfactory mesenchymal stromal cells co-expressing horizontal basal and ensheathing cell proteins in culture.Biomedica. 2020 Mar 1;40(1):72-88. doi: 10.7705/biomedica.4762. Biomedica. 2020. PMID: 32220165 Free PMC article.
-
Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology.J Mol Histol. 2007 Dec;38(6):581-99. doi: 10.1007/s10735-007-9141-2. Epub 2007 Sep 13. J Mol Histol. 2007. PMID: 17851769 Review.
-
Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license.J Comp Neurol. 2017 Mar 1;525(4):1034-1054. doi: 10.1002/cne.24105. Epub 2016 Sep 27. J Comp Neurol. 2017. PMID: 27560601 Free PMC article. Review.
Cited by
-
RNAseq analysis of olfactory neuroepithelium cytological samples in individuals with Down syndrome compared to euploid controls: a pilot study.Neurol Sci. 2023 Mar;44(3):919-930. doi: 10.1007/s10072-022-06500-2. Epub 2022 Nov 17. Neurol Sci. 2023. PMID: 36394661 Free PMC article.
-
Translational potential of olfactory mucosa for the study of neuropsychiatric illness.Transl Psychiatry. 2015 Mar 17;5(3):e527. doi: 10.1038/tp.2014.141. Transl Psychiatry. 2015. PMID: 25781226 Free PMC article. Review.
-
Retroviral lineage analysis reveals dual contribution from ectodermal placodes and neural crest cells to avian olfactory sensory and GnRH neurons.Nat Sci (Weinh). 2022 Jul;2(3):e20210037. doi: 10.1002/ntls.20210037. Epub 2022 May 25. Nat Sci (Weinh). 2022. PMID: 36311264 Free PMC article.
-
Nonintegrin laminin receptor precursor protein is expressed on olfactory stem and progenitor cells.J Comp Neurol. 2007 May 20;502(3):367-81. doi: 10.1002/cne.21328. J Comp Neurol. 2007. PMID: 17366606 Free PMC article.
-
Cyclophosphamide has Long-Term Effects on Proliferation in Olfactory Epithelia.Chem Senses. 2020 Mar 25;45(2):97-109. doi: 10.1093/chemse/bjz075. Chem Senses. 2020. PMID: 31844905 Free PMC article.
References
-
- Adams JC, Watt FM (1990) Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell 63: 425-435. - PubMed
-
- Aiba T, Mori J, Nakai Y (1993) Nerve growth factor (NGF) and its receptor in rat olfactory epithelium. Acta Otolaryngol Suppl (Stockh) 506: 37-40. - PubMed
-
- Arkin S, Naprstek B, Guarini L, Ferrone S, Lipton JM (1991) Expression of intercellular adhesion molecule-1 (CD54) on hematopoietic progenitors. Blood 77: 948-953. - PubMed
-
- Au E, Roskams AJ (2003) Olfactory ensheathing cells of the lamina propria in vivo and in vitro Glia 41: 224-236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
