A regulatory role for Sec tRNA[Ser]Sec in selenoprotein synthesis
- PMID: 15208449
- PMCID: PMC1370604
- DOI: 10.1261/rna.7370104
A regulatory role for Sec tRNA[Ser]Sec in selenoprotein synthesis
Abstract
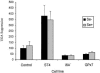
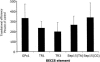
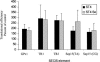
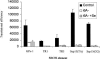
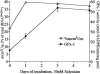
Selenium is biologically active through the functions of selenoproteins that contain the amino acid selenocysteine. This amino acid is translated in response to in-frame UGA codons in mRNAs that include a SECIS element in its 3' untranslated region, and this process requires a unique tRNA, referred to as tRNA([Ser]Sec). The translation of UGA as selenocysteine, rather than its use as a termination signal, is a candidate restriction point for the regulation of selenoprotein synthesis by selenium. A specialized reporter construct was used that permits the evaluation of SECIS-directed UGA translation to examine mechanisms of the regulation of selenoprotein translation. Using SECIS elements from five different selenoprotein mRNAs, UGA translation was quantified in response to selenium supplementation and alterations in tRNA([Ser]Sec) levels and isoform distributions. Although each of the evaluated SECIS elements exhibited differences in their baseline activities, each was stimulated to a similar extent by increased selenium or tRNA([Ser]Sec) levels and was inhibited by diminished levels of the methylated isoform of tRNA([Ser]Sec) achieved using a dominant-negative acting mutant tRNA([Ser]Sec). tRNA([Ser]Sec) was found to be limiting for UGA translation under conditions of high selenoprotein mRNA in both a transient reporter assay and in cells with elevated GPx-1 mRNA. This and data indicating increased amounts of the methylated isoform of tRNA([Ser]Sec) during selenoprotein translation indicate that it is this isoform that is translationally active and that selenium-induced tRNA methylation is a mechanism of regulation of the synthesis of selenoproteins.
Figures
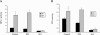
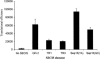

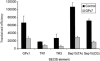
Similar articles
-
A recoding element that stimulates decoding of UGA codons by Sec tRNA[Ser]Sec.RNA. 2007 Jun;13(6):912-20. doi: 10.1261/rna.473907. Epub 2007 Apr 24. RNA. 2007. PMID: 17456565 Free PMC article.
-
High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes.J Mol Biol. 1999 Oct 8;292(5):1003-16. doi: 10.1006/jmbi.1999.3085. J Mol Biol. 1999. PMID: 10512699
-
A Versatile Strategy to Reduce UGA-Selenocysteine Recoding Efficiency of the Ribosome Using CRISPR-Cas9-Viral-Like-Particles Targeting Selenocysteine-tRNA[Ser]Sec Gene.Cells. 2019 Jun 11;8(6):574. doi: 10.3390/cells8060574. Cells. 2019. PMID: 31212706 Free PMC article.
-
Update on selenoprotein biosynthesis.Antioxid Redox Signal. 2015 Oct 1;23(10):775-94. doi: 10.1089/ars.2015.6391. Epub 2015 Aug 25. Antioxid Redox Signal. 2015. PMID: 26154496 Review.
-
Selenium. Role of the essential metalloid in health.Met Ions Life Sci. 2013;13:499-534. doi: 10.1007/978-94-007-7500-8_16. Met Ions Life Sci. 2013. PMID: 24470102 Free PMC article. Review.
Cited by
-
Potential role and therapeutic implications of glutathione peroxidase 4 in the treatment of Alzheimer's disease.Neural Regen Res. 2025 Mar 1;20(3):613-631. doi: 10.4103/NRR.NRR-D-23-01343. Epub 2024 Mar 1. Neural Regen Res. 2025. PMID: 38886929 Free PMC article.
-
Modeling and gene knockdown to assess the contribution of nonsense-mediated decay, premature termination, and selenocysteine insertion to the selenoprotein hierarchy.RNA. 2016 Jul;22(7):1076-84. doi: 10.1261/rna.055749.115. Epub 2016 May 20. RNA. 2016. PMID: 27208313 Free PMC article.
-
Nonsense-mediated decay factors are involved in the regulation of selenoprotein mRNA levels during selenium deficiency.RNA. 2014 Aug;20(8):1248-56. doi: 10.1261/rna.043463.113. Epub 2014 Jun 19. RNA. 2014. PMID: 24947499 Free PMC article.
-
tRNA modification enzyme-dependent redox homeostasis regulates synapse formation and memory.Proc Natl Acad Sci U S A. 2024 Nov 12;121(46):e2317864121. doi: 10.1073/pnas.2317864121. Epub 2024 Nov 4. Proc Natl Acad Sci U S A. 2024. PMID: 39495910 Free PMC article.
-
Different Forms of Selenoprotein M Differentially Affect Aβ Aggregation and ROS Generation.Int J Mol Sci. 2013 Feb 25;14(3):4385-99. doi: 10.3390/ijms14034385. Int J Mol Sci. 2013. PMID: 23439548 Free PMC article.
References
-
- Arner, E.S., Zhong, L., and Holmgren, A. 1999. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Meth. Enzymol. 300: 226–239. - PubMed
-
- Behne, D. and Kyriakopoulos, A. 1993. Effects of dietary selenium on the tissue concentrations of type I iodothyronine 5′-deiodinase and other selenoproteins. Am. J. Clin. Nutr. 57: 310S–312S. - PubMed
-
- Bright, R.K., Vocke, C.D., Emmert-Buck, M.R., Duray, P.H., Solomon, D., Fetsch, P., Rhim, J.S., Linehan, W.M., and Topalian, S.L. 1997. Generation and genetic characterization of immortal human prostate epithelial cell lines derived from primary cancer specimens. Cancer Res. 57: 995–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
