A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin
- PMID: 15199184
- PMCID: PMC454183
- DOI: 10.1073/pnas.0402829101
A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin
Abstract
The obligate intracellular bacterium Chlamydia trachomatis rapidly induces its own entry into host cells. Initial attachment is mediated by electrostatic interactions to heparan sulfate moieties on the host cell, followed by irreversible binding to an unknown secondary receptor. This secondary binding leads to the recruitment of actin to the site of attachment, formation of an actin-rich, pedestal-like structure, and finally internalization of the bacteria. How chlamydiae induce this process is unknown. We have identified a high-molecular-mass tyrosine-phosphorylated protein that is rapidly phosphorylated on attachment to the host cell. Immunoelectron microscopy studies revealed that this tyrosine-phosphorylated protein is localized to the cytoplasmic face of the plasma membrane at the site of attachment of surface-associated chlamydiae. The phosphoprotein was isolated by immunoprecipitation with the antiphosphotyrosine antibody 4G10 and identified as the chlamydial protein CT456, a hypothetical protein with unknown function. The chlamydial protein (Tarp) appears to be translocated into the host cell by type III secretion because it is exported in a Yersinia heterologous expression assay. Phosphotyrosine signaling across the plasma membrane preceded the recruitment of actin to the site of chlamydial attachment and may represent the initial signal transduced from pathogen to the host cell. These results suggest that C. trachomatis internalization is mediated by a chlamydial type III-secreted effector protein.
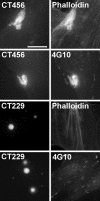
Figures





Comment in
-
Tarp and Arp: How Chlamydia induces its own entry.Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):9947-8. doi: 10.1073/pnas.0403633101. Epub 2004 Jun 29. Proc Natl Acad Sci U S A. 2004. PMID: 15226494 Free PMC article. No abstract available.
Similar articles
-
Tyrosine phosphorylation of the chlamydial effector protein Tarp is species specific and not required for recruitment of actin.Infect Immun. 2005 Jul;73(7):3860-8. doi: 10.1128/IAI.73.7.3860-3868.2005. Infect Immun. 2005. PMID: 15972471 Free PMC article.
-
Chlamydial entry involves TARP binding of guanine nucleotide exchange factors.PLoS Pathog. 2008 Mar;4(3):e1000014. doi: 10.1371/journal.ppat.1000014. PLoS Pathog. 2008. PMID: 18383626 Free PMC article.
-
Chlamydial TARP is a bacterial nucleator of actin.Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15599-604. doi: 10.1073/pnas.0603044103. Epub 2006 Oct 6. Proc Natl Acad Sci U S A. 2006. PMID: 17028176 Free PMC article.
-
Pathogenic Puppetry: Manipulation of the Host Actin Cytoskeleton by Chlamydia trachomatis.Int J Mol Sci. 2019 Dec 21;21(1):90. doi: 10.3390/ijms21010090. Int J Mol Sci. 2019. PMID: 31877733 Free PMC article. Review.
-
Chlamydia effector proteins and new insights into chlamydial cellular microbiology.Curr Opin Microbiol. 2008 Feb;11(1):53-9. doi: 10.1016/j.mib.2008.01.003. Epub 2008 Mar 4. Curr Opin Microbiol. 2008. PMID: 18299248 Review.
Cited by
-
Chlamydia trachomatis Tarp cooperates with the Arp2/3 complex to increase the rate of actin polymerization.Biochem Biophys Res Commun. 2012 Apr 20;420(4):816-21. doi: 10.1016/j.bbrc.2012.03.080. Epub 2012 Mar 23. Biochem Biophys Res Commun. 2012. PMID: 22465117 Free PMC article.
-
c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains.J Clin Invest. 2012 Apr;122(4):1553-66. doi: 10.1172/JCI61143. Epub 2012 Mar 1. J Clin Invest. 2012. PMID: 22378042 Free PMC article.
-
Systematic analysis of phosphotyrosine antibodies recognizing single phosphorylated EPIYA-motifs in CagA of East Asian-type Helicobacter pylori strains.BMC Microbiol. 2016 Sep 2;16(1):201. doi: 10.1186/s12866-016-0820-6. BMC Microbiol. 2016. PMID: 27590005 Free PMC article.
-
Genome-wide identification of Chlamydia trachomatis antigens associated with trachomatous trichiasis.Invest Ophthalmol Vis Sci. 2012 May 14;53(6):2551-9. doi: 10.1167/iovs.11-9212. Print 2012 May. Invest Ophthalmol Vis Sci. 2012. PMID: 22427578 Free PMC article.
-
In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract.Infect Immun. 2015 May;83(5):1881-92. doi: 10.1128/IAI.03158-14. Epub 2015 Feb 23. Infect Immun. 2015. PMID: 25712926 Free PMC article.
References
-
- Schachter, J. (1999) in Chlamydia: Intracellular Biology, Pathogenesis, and Immunity, ed. Stephens, R. S. (Am. Soc. Microbiol., Washington, DC), pp. 139-169.
-
- Hackstadt, T. (1999) in Chlamydia: Intracellular Biology, Pathogenesis, and Immunity., ed. Stephens, R. S. (Am. Soc. Microbiol., Washington, DC), pp. 101-138.
-
- Zhang, J. P. & Stephens, R. S. (1992) Cell 69, 861-869. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

