Stimulus-specific defect in the phagocytic pathways of annexin 1 null macrophages
- PMID: 15197108
- PMCID: PMC1575068
- DOI: 10.1038/sj.bjp.0705858
Stimulus-specific defect in the phagocytic pathways of annexin 1 null macrophages
Abstract
The role of the glucocorticoid-regulated protein annexin 1 during the process of phagocytosis has been studied using annexin 1 null peritoneal macrophages. Wild type and annexin 1 null macrophages were incubated with several distinct phagocytic targets. No differences were observed in rate or the maximal response with respect to IgG complexes or opsonised zymosan phagocytosis, as assessed by monitoring the production of reactive oxygen species. When annexin 1 null macrophages were incubated with non-opsonised zymosan particles, they exhibited impaired generation of reactive oxygen species, which was linked to a defect in binding of cells to the particles, as determined with fluorescent zymosan. This phenomenon was further confirmed by electron microscopy analysis, where annexin 1 null macrophages internalised fewer non-opsonised zymosan particles. Specific alterations in macrophage plasma membrane markers were observed in the annexin 1 null cells. Whereas no differences in dectin-1 and FcgammaR II/III expression were measured between the two genotypes, decreased membrane CD11b and F4/80 levels were measured selectively in macrophages lacking annexin 1. These cells also responded with an enhanced release of PGE(2) and COX-2 protein expression following addition of the soluble stimulants, LPS and heat-activated IgG. In conclusion, these results suggest that participation of endogenous annexin 1 during zymosan phagocytosis is critical and that this protein plays a tonic inhibitory role during macrophage activation.
Figures
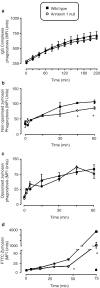
 and ▴, respectively. For all panels, data are mean ± s.e.m. of n=4 experiments performed with n=3 mice each. *P<0.05 vs respective WT value.
and ▴, respectively. For all panels, data are mean ± s.e.m. of n=4 experiments performed with n=3 mice each. *P<0.05 vs respective WT value.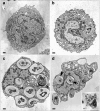

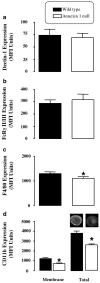

Similar articles
-
Macrophage biology in the Anx-A1-/- mouse.Prostaglandins Leukot Essent Fatty Acids. 2005 Feb;72(2):95-103. doi: 10.1016/j.plefa.2004.10.008. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 15626592 Review.
-
Desensitization of macrophage oxygen metabolism on immobilized ligands: different effect of immunoglobulin G and complement.J Immunol. 1987 Jun 15;138(12):4366-73. J Immunol. 1987. PMID: 2953806
-
Impaired phagocytic mechanism in annexin 1 null macrophages.Br J Pharmacol. 2006 Jun;148(4):469-77. doi: 10.1038/sj.bjp.0706730. Epub 2006 Apr 24. Br J Pharmacol. 2006. PMID: 16633358 Free PMC article.
-
PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages.J Leukoc Biol. 2004 Apr;75(4):649-56. doi: 10.1189/jlb.0803371. Epub 2004 Jan 14. J Leukoc Biol. 2004. PMID: 14726497
-
Zymosan phagocytosis by mouse peritoneal macrophages is increased by apoHDL- and not by intact HDL-covered particles.Braz J Med Biol Res. 2000 Mar;33(3):313-6. doi: 10.1590/s0100-879x2000000300009. Braz J Med Biol Res. 2000. PMID: 10719383 Review.
Cited by
-
Cromoglycate drugs suppress eicosanoid generation in U937 cells by promoting the release of Anx-A1.Biochem Pharmacol. 2009 Jun 15;77(12):1814-26. doi: 10.1016/j.bcp.2009.03.010. Epub 2009 Mar 24. Biochem Pharmacol. 2009. PMID: 19428336 Free PMC article.
-
The anti-inflammatory Annexin A1 induces the clearance and degradation of the amyloid-β peptide.J Neuroinflammation. 2016 Sep 2;13(1):234. doi: 10.1186/s12974-016-0692-6. J Neuroinflammation. 2016. PMID: 27590054 Free PMC article.
-
Matrix metalloproteinase-12 as an endogenous resolution promoting factor following myocardial infarction.Pharmacol Res. 2018 Nov;137:252-258. doi: 10.1016/j.phrs.2018.10.026. Epub 2018 Oct 28. Pharmacol Res. 2018. PMID: 30394317 Free PMC article. Review.
-
Annexin A1 regulates neutrophil clearance by macrophages in the mouse bone marrow.FASEB J. 2012 Jan;26(1):387-96. doi: 10.1096/fj.11-182089. Epub 2011 Sep 28. FASEB J. 2012. PMID: 21957127 Free PMC article.
-
Endogenous Annexin-A1 Regulates Haematopoietic Stem Cell Mobilisation and Inflammatory Response Post Myocardial Infarction in Mice In Vivo.Sci Rep. 2017 Nov 30;7(1):16615. doi: 10.1038/s41598-017-16317-1. Sci Rep. 2017. PMID: 29192208 Free PMC article.
References
-
- ADEREM A., UNDERHILL D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. - PubMed
-
- AMBROSE M.P., BAHNS C.L., HUNNINGHAKE G.W. Lipocortin I production by human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1992;6:17–21. - PubMed
-
- ARUR S., UCHE U.E., REZAUL K., FONG M., SCRANTON V., COWAN A.E., MOHLER W., HAN D.K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell. 2003;4:587–598. - PubMed
-
- AVNI O., PUR Z., YEFENOF E., BANIYASH M. Complement receptor 3 of macrophages is associated with galectin-1-like protein. J. Immunol. 1998;160:6151–6158. - PubMed
-
- BECKER J., GRASSO R.J. Suppression of yeast ingestion by dexamethasone in macrophage cultures: evidence for a steroid-induced phagocytosis inhibitory protein. Int. J. Immunopharmacol. 1988;10:325–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

