The phosphatidylserine receptor from Hydra is a nuclear protein with potential Fe(II) dependent oxygenase activity
- PMID: 15193161
- PMCID: PMC442123
- DOI: 10.1186/1471-2121-5-26
The phosphatidylserine receptor from Hydra is a nuclear protein with potential Fe(II) dependent oxygenase activity
Abstract
Background: Apoptotic cell death plays an essential part in embryogenesis, development and maintenance of tissue homeostasis in metazoan animals. The culmination of apoptosis in vivo is the phagocytosis of cellular corpses. One morphological characteristic of cells undergoing apoptosis is loss of plasma membrane phospholipid asymmetry and exposure of phosphatidylserine on the outer leaflet. Surface exposure of phosphatidylserine is recognised by a specific receptor (phosphatidylserine receptor, PSR) and is required for phagocytosis of apoptotic cells by macrophages and fibroblasts.
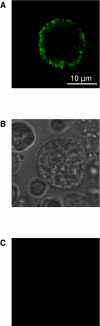
Results: We have cloned the PSR receptor from Hydra in order to investigate its function in this early metazoan. Bioinformatic analysis of the Hydra PSR protein structure revealed the presence of three nuclear localisation signals, an AT-hook like DNA binding motif and a putative 2-oxoglutarate (2OG)-and Fe(II)-dependent oxygenase activity. All of these features are conserved from human PSR to Hydra PSR. Expression of GFP tagged Hydra PSR in hydra cells revealed clear nuclear localisation. Deletion of one of the three NLS sequences strongly diminished nuclear localisation of the protein. Membrane localisation was never detected.
Conclusions: Our results suggest that Hydra PSR is a nuclear 2-oxoglutarate (2OG)-and Fe(II)-dependent oxygenase. This is in contrast with the proposed function of Hydra PSR as a cell surface receptor involved in the recognition of apoptotic cells displaying phosphatidylserine on their surface. The conservation of the protein from Hydra to human infers that our results also apply to PSR from higher animals.
Figures

Similar articles
-
Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12.Science. 2003 Nov 28;302(5650):1563-6. doi: 10.1126/science.1087641. Science. 2003. PMID: 14645848
-
Phosphatidylserine receptor is required for the engulfment of dead apoptotic cells and for normal embryonic development in zebrafish.Development. 2004 Nov;131(21):5417-27. doi: 10.1242/dev.01409. Epub 2004 Oct 6. Development. 2004. PMID: 15469976
-
A receptor for phosphatidylserine-specific clearance of apoptotic cells.Nature. 2000 May 4;405(6782):85-90. doi: 10.1038/35011084. Nature. 2000. PMID: 10811223
-
If phosphatidylserine is the death knell, a new phosphatidylserine-specific receptor is the bellringer.Cell Death Differ. 2001 Jun;8(6):582-7. doi: 10.1038/sj.cdd.4400856. Cell Death Differ. 2001. PMID: 11536008 Review.
-
New insights into the mechanism for clearance of apoptotic cells.Bioessays. 2000 Oct;22(10):878-81. doi: 10.1002/1521-1878(200010)22:10<878::AID-BIES2>3.0.CO;2-J. Bioessays. 2000. PMID: 10984713 Review.
Cited by
-
Trembley's polyps go transgenic.Proc Natl Acad Sci U S A. 2006 Apr 25;103(17):6415-6. doi: 10.1073/pnas.0601983103. Epub 2006 Apr 17. Proc Natl Acad Sci U S A. 2006. PMID: 16618934 Free PMC article. No abstract available.
-
Phylogenetic and genomic analyses of the ribosomal oxygenases Riox1 (No66) and Riox2 (Mina53) provide new insights into their evolution.BMC Evol Biol. 2018 Jun 19;18(1):96. doi: 10.1186/s12862-018-1215-0. BMC Evol Biol. 2018. PMID: 29914368 Free PMC article.
-
Jmjd6, a JmjC Dioxygenase with Many Interaction Partners and Pleiotropic Functions.Front Genet. 2017 Mar 16;8:32. doi: 10.3389/fgene.2017.00032. eCollection 2017. Front Genet. 2017. PMID: 28360925 Free PMC article. Review.
-
JMJD6 and U2AF65 co-regulate alternative splicing in both JMJD6 enzymatic activity dependent and independent manner.Nucleic Acids Res. 2017 Apr 7;45(6):3503-3518. doi: 10.1093/nar/gkw1144. Nucleic Acids Res. 2017. PMID: 27899633 Free PMC article.
-
Hide and seek: the secret identity of the phosphatidylserine receptor.J Biol. 2004;3(4):14. doi: 10.1186/jbiol14. Epub 2004 Sep 23. J Biol. 2004. PMID: 15453906 Free PMC article. Review.
References
-
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. - DOI - PMC - PubMed
-
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

