The XMAP215-family protein DdCP224 is required for cortical interactions of microtubules
- PMID: 15186508
- PMCID: PMC434496
- DOI: 10.1186/1471-2121-5-24
The XMAP215-family protein DdCP224 is required for cortical interactions of microtubules
Abstract
Background: Interactions of peripheral microtubule tips with the cell cortex are of crucial importance for nuclear migration, spindle orientation, centrosome positioning and directional cell movement. Microtubule plus end binding proteins are thought to mediate interactions of microtubule tips with cortical actin and membrane proteins in a dynein-dependent manner. XMAP215-family proteins are main regulators of microtubule plus end dynamics but so far they have not been implicated in the interactions of microtubule tips with the cell cortex.
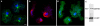
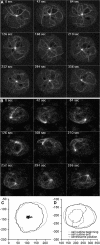
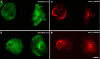
Results: Here we show that overexpression of an N-terminal fragment of DdCP224, the Dictyostelium XMAP215 homologue, caused a collapse of the radial microtubule cytoskeleton, whereby microtubules lost contact with the cell cortex and were dragged behind like a comet tail of an unusually motile centrosome. This phenotype was indistinguishable from mutants overexpressing fragments of the dynein heavy chain or intermediate chain. Moreover, it was accompanied by dispersal of the Golgi apparatus and reduced cortical localization of the dynein heavy chain indicating a disrupted dynein/dynactin interaction. The interference of DdCP224 with cortical dynein function is strongly supported by the observations that DdCP224 and its N-terminal fragment colocalize with dynein and coimmunoprecipitate with dynein and dynactin.
Conclusions: Our data show that XMAP215-like proteins are required for the interaction of microtubule plus ends with the cell cortex in interphase cells and strongly suggest that this function is mediated by dynein.
Figures
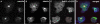

Similar articles
-
Centrosomal microtubule plus end tracking proteins and their role in Dictyostelium cell dynamics.J Muscle Res Cell Motil. 2002;23(7-8):621-30. doi: 10.1023/a:1024450922609. J Muscle Res Cell Motil. 2002. PMID: 12952061 Review.
-
Dictyostelium LIS1 is a centrosomal protein required for microtubule/cell cortex interactions, nucleus/centrosome linkage, and actin dynamics.Mol Biol Cell. 2005 Jun;16(6):2759-71. doi: 10.1091/mbc.e05-01-0069. Epub 2005 Mar 30. Mol Biol Cell. 2005. PMID: 15800059 Free PMC article.
-
Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium.J Cell Biol. 1999 Dec 13;147(6):1261-74. doi: 10.1083/jcb.147.6.1261. J Cell Biol. 1999. PMID: 10601339 Free PMC article.
-
Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number.Mol Biol Cell. 2003 Oct;14(10):4067-74. doi: 10.1091/mbc.e03-04-0242. Epub 2003 Jun 13. Mol Biol Cell. 2003. PMID: 14517319 Free PMC article.
-
[Dynein and dynactin as organizers of the system of cell microtubules].Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian.
Cited by
-
The Dictyostelium Centrosome.Cells. 2021 Oct 5;10(10):2657. doi: 10.3390/cells10102657. Cells. 2021. PMID: 34685637 Free PMC article. Review.
-
Force balances between interphase centrosomes as revealed by laser ablation.Mol Biol Cell. 2019 Jul 1;30(14):1705-1715. doi: 10.1091/mbc.E19-01-0034. Epub 2019 May 8. Mol Biol Cell. 2019. PMID: 31067156 Free PMC article.
-
13 Plus 1: A 30-Year Perspective on Microtubule-Based Motility in Dictyostelium.Cells. 2020 Feb 25;9(3):528. doi: 10.3390/cells9030528. Cells. 2020. PMID: 32106406 Free PMC article. Review.
-
The microtubule-associated protein tumor overexpressed gene/cytoskeleton-associated protein 5 is necessary for myelin basic protein expression in oligodendrocytes.J Neurosci. 2007 Jul 18;27(29):7654-62. doi: 10.1523/JNEUROSCI.0203-07.2007. J Neurosci. 2007. PMID: 17634360 Free PMC article.
-
Aspergillus nidulans Dis1/XMAP215 protein AlpA localizes to spindle pole bodies and microtubule plus ends and contributes to growth directionality.Eukaryot Cell. 2007 Mar;6(3):555-62. doi: 10.1128/EC.00266-06. Epub 2007 Jan 19. Eukaryot Cell. 2007. PMID: 17237365 Free PMC article.
References
-
- Ohkura H, Garcia MA, Toda T. Dis1/TOG universal microtubule adaptors - one MAP for all? J Cell Sci. 2001;114:3805–3812. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

