Short duration of elevated vIRF-1 expression during lytic replication of human herpesvirus 8 limits its ability to block antiviral responses induced by alpha interferon in BCBL-1 cells
- PMID: 15163753
- PMCID: PMC416518
- DOI: 10.1128/JVI.78.12.6621-6635.2004
Short duration of elevated vIRF-1 expression during lytic replication of human herpesvirus 8 limits its ability to block antiviral responses induced by alpha interferon in BCBL-1 cells
Abstract
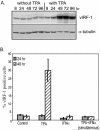
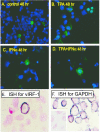
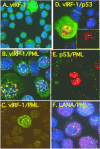
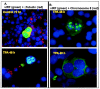
Human herpesvirus 8 (HHV-8) encodes multiple proteins that disrupt the host antiviral response, including viral interferon (IFN) regulatory factor 1 (vIRF-1). The product of the vIRF-1 gene blocks responses to IFN when overexpressed by transfection, but the functional consequence of vIRF-1 that is expressed during infection with HHV-8 is not known. These studies demonstrate that BCBL-1 cells that were latently infected with HHV-8 expressed low levels of vIRF-1 that were associated with PML bodies, whereas much higher levels of vIRF-1 were transiently expressed during the lytic phase of HHV-8 replication. The low levels of vIRF-1 that were associated with PML bodies were insufficient to block alpha IFN (IFN-alpha)-induced alterations in gene expression, whereas cells that expressed high levels of vIRF-1 were resistant to some changes induced by IFN-alpha, including the expression of the double-stranded-RNA-activated protein kinase. High levels of vIRF-1 were expressed for only a short period during the lytic cascade, so many cells with HHV-8 in the lytic phase responded to IFN-alpha with increased expression of antiviral genes and enhanced apoptosis. Furthermore, the production of infectious virus was severely compromised when IFN-alpha was present early during the lytic cascade. These studies indicate that the transient expression of high levels of vIRF-1 is inadequate to subvert many of the antiviral effects of IFN-alpha so that IFN-alpha can effectively induce apoptosis and block production of infectious virus when present early in the lytic cascade of HHV-8.
Figures

Similar articles
-
Human Herpesvirus 8 Interferon Regulatory Factors 1 and 3 Mediate Replication and Latency Activities via Interactions with USP7 Deubiquitinase.J Virol. 2018 Mar 14;92(7):e02003-17. doi: 10.1128/JVI.02003-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343584 Free PMC article.
-
Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase.J Virol. 2001 Mar;75(5):2345-52. doi: 10.1128/JVI.75.5.2345-2352.2001. J Virol. 2001. PMID: 11160738 Free PMC article.
-
Inhibition of infectious human herpesvirus 8 production by gamma interferon and alpha interferon in BCBL-1 cells.J Gen Virol. 2004 Oct;85(Pt 10):2779-2787. doi: 10.1099/vir.0.80214-0. J Gen Virol. 2004. PMID: 15448338
-
On the role of IRF in host defense.J Interferon Cytokine Res. 2002 Jan;22(1):59-71. doi: 10.1089/107999002753452665. J Interferon Cytokine Res. 2002. PMID: 11846976 Review.
-
Kaposi sarcoma herpesvirus-encoded interferon regulator factors.Curr Top Microbiol Immunol. 2007;312:185-209. doi: 10.1007/978-3-540-34344-8_7. Curr Top Microbiol Immunol. 2007. PMID: 17089798 Review.
Cited by
-
In vivo electroporation and non-protein based screening assays to identify antibodies against native protein conformations.Hybridoma (Larchmt). 2011 Oct;30(5):409-18. doi: 10.1089/hyb.2010.0120. Hybridoma (Larchmt). 2011. PMID: 22008067 Free PMC article.
-
Identification of Kaposi Sarcoma Herpesvirus (KSHV) vIRF1 Protein as a Novel Interaction Partner of Human Deubiquitinase USP7.J Biol Chem. 2016 Mar 18;291(12):6281-91. doi: 10.1074/jbc.M115.710632. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786098 Free PMC article.
-
Direct and biologically significant interactions of human herpesvirus 8 interferon regulatory factor 1 with STAT3 and Janus kinase TYK2.PLoS Pathog. 2023 Nov 20;19(11):e1011806. doi: 10.1371/journal.ppat.1011806. eCollection 2023 Nov. PLoS Pathog. 2023. PMID: 37983265 Free PMC article.
-
The mitophagy receptor NIX induces vIRF-1 oligomerization and interaction with GABARAPL1 for the promotion of HHV-8 reactivation-induced mitophagy.PLoS Pathog. 2023 Jul 17;19(7):e1011548. doi: 10.1371/journal.ppat.1011548. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37459327 Free PMC article.
-
Human Herpesvirus 8 Interferon Regulatory Factors 1 and 3 Mediate Replication and Latency Activities via Interactions with USP7 Deubiquitinase.J Virol. 2018 Mar 14;92(7):e02003-17. doi: 10.1128/JVI.02003-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29343584 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

