Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency
- PMID: 15163750
- PMCID: PMC416549
- DOI: 10.1128/JVI.78.12.6585-6594.2004
Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency
Abstract
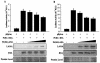
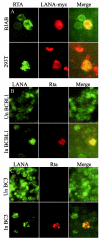
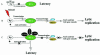
Like other herpesviruses, Kaposi's sarcoma-associated herpesvirus (KSHV, also designated human herpesvirus 8) can establish a latent infection in the infected host. During latency a small number of genes are expressed. One of those genes encodes latency-associated nuclear antigen (LANA), which is constitutively expressed in cells during latent as well as lytic infection. LANA has previously been shown to be important for the establishment of latent episome maintenance through tethering of the viral genome to the host chromosomes. Under specific conditions, KSHV can undergo lytic replication, with the production of viral progeny. The immediate-early Rta, encoded by open reading frame 50 of KSHV, has been shown to play a critical role in switching from viral latent replication to lytic replication. Overexpression of Rta from a heterologous promoter is sufficient for driving KSHV lytic replication and the production of viral progeny. In the present study, we show that LANA down-modulates Rta's promoter activity in transient reporter assays, thus repressing Rta-mediated transactivation. This results in a decrease in the production of KSHV progeny virions. We also found that LANA interacts physically with Rta both in vivo and in vitro. Taken together, our results demonstrate that LANA can inhibit viral lytic replication by inhibiting expression as well as antagonizing the function of Rta. This suggests that LANA may play a critical role in maintaining latency by controlling the switch between viral latency and lytic replication.
Figures

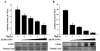




Similar articles
-
Induction of Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency.J Virol. 2005 Jun;79(12):7453-65. doi: 10.1128/JVI.79.12.7453-7465.2005. J Virol. 2005. PMID: 15919901 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen modulates K1 expression through its cis-acting elements within the terminal repeats.J Virol. 2006 Apr;80(7):3445-58. doi: 10.1128/JVI.80.7.3445-3458.2006. J Virol. 2006. PMID: 16537612 Free PMC article.
-
Full-Length Isoforms of Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Accumulate in the Cytoplasm of Cells Undergoing the Lytic Cycle of Replication.J Virol. 2017 Nov 30;91(24):e01532-17. doi: 10.1128/JVI.01532-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978712 Free PMC article.
-
KSHV reactivation and novel implications of protein isomerization on lytic switch control.Viruses. 2015 Jan 12;7(1):72-109. doi: 10.3390/v7010072. Viruses. 2015. PMID: 25588053 Free PMC article. Review.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
The Modulation of Apoptotic Pathways by Gammaherpesviruses.Front Microbiol. 2016 Apr 27;7:585. doi: 10.3389/fmicb.2016.00585. eCollection 2016. Front Microbiol. 2016. PMID: 27199919 Free PMC article. Review.
-
Molecular biology of KSHV in relation to AIDS-associated oncogenesis.Cancer Treat Res. 2007;133:69-127. doi: 10.1007/978-0-387-46816-7_3. Cancer Treat Res. 2007. PMID: 17672038 Free PMC article. Review.
-
Immune evasion in Kaposi's sarcoma-associated herpes virus associated oncogenesis.Semin Cancer Biol. 2008 Dec;18(6):423-36. doi: 10.1016/j.semcancer.2008.09.003. Epub 2008 Oct 2. Semin Cancer Biol. 2008. PMID: 18948197 Free PMC article. Review.
-
Kaposi's sarcoma-associated herpesvirus-encoded replication and transcription activator impairs innate immunity via ubiquitin-mediated degradation of myeloid differentiation factor 88.J Virol. 2015 Jan;89(1):415-27. doi: 10.1128/JVI.02591-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320320 Free PMC article.
-
Epigenetic Landscape of Kaposi's Sarcoma-Associated Herpesvirus Genome in Classic Kaposi's Sarcoma Tissues.PLoS Pathog. 2017 Jan 24;13(1):e1006167. doi: 10.1371/journal.ppat.1006167. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28118409 Free PMC article.
References
-
- Aguirre, A. J., and E. S. Robertson. 1999. Characterization of intertypic recombinants of the Epstein-Barr virus from the body-cavity-based lymphomas cell lines BC-1 and BC-2. Virology 264:359-369. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

