Involvement of Per-Arnt-Sim (PAS) kinase in the stimulation of preproinsulin and pancreatic duodenum homeobox 1 gene expression by glucose
- PMID: 15148392
- PMCID: PMC420392
- DOI: 10.1073/pnas.0307737101
Involvement of Per-Arnt-Sim (PAS) kinase in the stimulation of preproinsulin and pancreatic duodenum homeobox 1 gene expression by glucose
Abstract
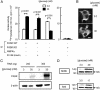
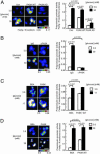
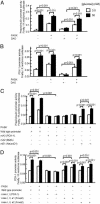
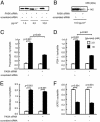
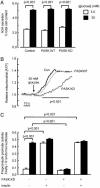
Per-Arnt-Sim (PAS) domain-containing kinases are common in prokaryotes, but a mammalian counterpart has only recently been described. Although the PAS domain of the mammalian PAS kinase (PASK) is closely related to the bacterial oxygen sensor FixL, it is unclear whether PASK activity is changed in mammalian cells in response to nutrients and might therefore contribute to signal transduction by these or other stimuli. Here, we show that elevated glucose concentrations rapidly increase PASK activity in pancreatic islet beta cells, an event followed by the accumulation of both PASK mRNA and protein. Demonstrating a physiological role for PASK activation, comicroinjection into clonal beta cells of cDNA encoding wild-type PASK, or PASK protein itself, mimics the induction of preproinsulin promoter activity by high glucose concentrations. Conversely, anti-PASK antibodies block promoter activation by the sugar, and the silencing of PASK expression by RNA interference suppresses the up-regulation by glucose of preproinsulin and pancreatic duodenum homeobox 1 gene expression, without affecting glucose-induced changes in the levels of mRNAs encoding glucokinase or uncoupling protein 2. We conclude that PASK is an important metabolic sensor in nutrient-sensitive mammalian cells and plays an unexpected role in the regulation of key genes involved in maintaining the differentiated phenotype of pancreatic beta cells.
Figures
Similar articles
-
Per-Arnt-Sim kinase regulates pancreatic duodenal homeobox-1 protein stability via phosphorylation of glycogen synthase kinase 3β in pancreatic β-cells.J Biol Chem. 2013 Aug 23;288(34):24825-33. doi: 10.1074/jbc.M113.495945. Epub 2013 Jul 12. J Biol Chem. 2013. PMID: 23853095 Free PMC article.
-
Per-arnt-sim (PAS) domain-containing protein kinase is downregulated in human islets in type 2 diabetes and regulates glucagon secretion.Diabetologia. 2011 Apr;54(4):819-27. doi: 10.1007/s00125-010-2010-7. Epub 2010 Dec 23. Diabetologia. 2011. PMID: 21181396 Free PMC article.
-
Human mutation within Per-Arnt-Sim (PAS) domain-containing protein kinase (PASK) causes basal insulin hypersecretion.J Biol Chem. 2011 Dec 23;286(51):44005-44014. doi: 10.1074/jbc.M111.254995. Epub 2011 Nov 7. J Biol Chem. 2011. PMID: 22065581 Free PMC article.
-
Per-Arnt-Sim Kinase (PASK): An Emerging Regulator of Mammalian Glucose and Lipid Metabolism.Nutrients. 2015 Sep 7;7(9):7437-50. doi: 10.3390/nu7095347. Nutrients. 2015. PMID: 26371032 Free PMC article. Review.
-
The role of PAS kinase in regulating energy metabolism.IUBMB Life. 2008 Apr;60(4):204-9. doi: 10.1002/iub.32. IUBMB Life. 2008. PMID: 18344204 Review.
Cited by
-
Protein Kinases in Obesity, and the Kinase-Targeted Therapy.Adv Exp Med Biol. 2024;1460:199-229. doi: 10.1007/978-3-031-63657-8_7. Adv Exp Med Biol. 2024. PMID: 39287853 Review.
-
Per-arnt-sim (PAS) domain kinase (PASK) as a regulator of glucagon secretion.Diabetologia. 2011 Apr;54(4):719-21. doi: 10.1007/s00125-011-2072-1. Epub 2011 Feb 17. Diabetologia. 2011. PMID: 21327866
-
Integrated analysis of recurrent properties of cancer genes to identify novel drivers.Genome Biol. 2013 May 29;14(5):R52. doi: 10.1186/gb-2013-14-5-r52. Genome Biol. 2013. PMID: 23718799 Free PMC article.
-
The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness.Biochim Biophys Acta. 2012 Dec;1826(2):272-96. doi: 10.1016/j.bbcan.2012.04.008. Epub 2012 May 10. Biochim Biophys Acta. 2012. PMID: 22579961 Free PMC article. Review.
-
Cell type-specific deletion in mice reveals roles for PAS kinase in insulin and glucagon production.Diabetologia. 2016 Sep;59(9):1938-47. doi: 10.1007/s00125-016-4025-1. Epub 2016 Jun 24. Diabetologia. 2016. PMID: 27338626 Free PMC article.
References
-
- Wilson, W. A. & Roach, P. J. (2002) Cell 111, 155-158. - PubMed
-
- Hofer, T., Spielmann, P., Stengel, P., Stier, B., Katschinski, D. M., Desbaillets, I., Gassmann, M. & Wenger, R. H. (2001) Biochem. Biophys. Res. Commun. 288, 757-764. - PubMed
-
- David, M., Daveran, M. L., Batut, J., Dedieu, A., Domergue, O., Ghai, J., Hertig, C., Boistard, P. & Kahn, D. (1988) Cell 54, 671-683. - PubMed
-
- Gu, Y. Z., Hogenesch, J. B. & Bradfield, C. A. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 519-561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

