Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling
- PMID: 15143188
- PMCID: PMC416405
- DOI: 10.1128/MCB.24.11.4968-4978.2004
Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling
Abstract
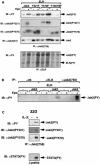
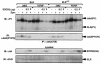
Jak family tyrosine kinases mediate signaling by cytokine receptors to regulate diverse biological processes. Although Jak2 and other Jak kinase family members are phosphorylated on numerous sites during cytokine signaling, the identity and function of most of these sites remains unknown. Using tandem mass spectroscopic analysis of activated Jak2 protein from intact cells, we identified Tyr(221) and Tyr(570) as novel sites of Jak2 phosphorylation. Phosphorylation of both sites was stimulated by cytokine treatment of cultured cells, and this stimulation required Jak2 kinase activity. While we observed no gross alteration of signaling upon mutation of Tyr(221), Tyr(570) lies within the inhibitory JH2 domain of Jak2, and mutation of this site (Jak2(Y570F)) results in constitutive Jak2-dependent signaling in the absence of cytokine stimulation and enhances and prolongs Jak2 activation during cytokine stimulation. Mutation of Tyr(570) does not alter the ability of SOCS3 to bind or inhibit Jak2, however. Thus, the phosphorylation of Tyr(570) in vivo inhibits Jak2-dependent signaling independently of SOCS3-mediated inhibition. This Tyr(570)-dependent mechanism of Jak2 inhibition likely represents an important mechanism by which cytokine function is regulated.
Figures





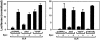
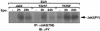
Similar articles
-
The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction.J Biol Chem. 2002 Dec 6;277(49):47954-63. doi: 10.1074/jbc.M205156200. Epub 2002 Sep 25. J Biol Chem. 2002. PMID: 12351625
-
Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase.Mol Endocrinol. 1996 Nov;10(11):1425-43. doi: 10.1210/mend.10.11.8923468. Mol Endocrinol. 1996. PMID: 8923468
-
Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain.Genes Cells. 1999 Jun;4(6):339-51. doi: 10.1046/j.1365-2443.1999.00263.x. Genes Cells. 1999. PMID: 10421843
-
JAK/STAT signal transduction: regulators and implication in hematological malignancies.Biochem Pharmacol. 2006 Mar 14;71(6):713-21. doi: 10.1016/j.bcp.2005.12.017. Epub 2006 Jan 19. Biochem Pharmacol. 2006. PMID: 16426581 Review.
-
Leptin receptor signaling and the regulation of mammalian physiology.Recent Prog Horm Res. 2004;59:287-304. doi: 10.1210/rp.59.1.287. Recent Prog Horm Res. 2004. PMID: 14749507 Review.
Cited by
-
Alternative TEL-JAK2 fusions associated with T-cell acute lymphoblastic leukemia and atypical chronic myelogenous leukemia dissected in zebrafish.Haematologica. 2012 Dec;97(12):1895-903. doi: 10.3324/haematol.2012.064659. Epub 2012 Jun 24. Haematologica. 2012. PMID: 22733019 Free PMC article.
-
Differential regulation of Janus kinase 3 (JAK3) in bovine preovulatory follicles and identification of JAK3 interacting proteins in granulosa cells.J Ovarian Res. 2016 Oct 28;9(1):71. doi: 10.1186/s13048-016-0280-5. J Ovarian Res. 2016. PMID: 27793176 Free PMC article.
-
Mechanistic Insights into Regulation of JAK2 Tyrosine Kinase.Front Endocrinol (Lausanne). 2018 Jan 5;8:361. doi: 10.3389/fendo.2017.00361. eCollection 2017. Front Endocrinol (Lausanne). 2018. PMID: 29379470 Free PMC article. Review.
-
Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity.Mol Cell Biol. 2004 Jun;24(11):4955-67. doi: 10.1128/MCB.24.11.4955-4967.2004. Mol Cell Biol. 2004. PMID: 15143187 Free PMC article.
-
Mechanistic insights into activation and SOCS3-mediated inhibition of myeloproliferative neoplasm-associated JAK2 mutants from biochemical and structural analyses.Biochem J. 2014 Mar 1;458(2):395-405. doi: 10.1042/BJ20131516. Biochem J. 2014. PMID: 24354892 Free PMC article.
References
-
- Banks, A. S., S. M. Davis, S. H. Bates, and M. G. Myers, Jr. 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275:14563-14572. - PubMed
-
- Bjorbaek, C., K. El Haschimi, J. D. Frantz, and J. S. Flier. 1999. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 274:30059-30065. - PubMed
-
- Bjorbak, C., H. J. Lavery, S. H. Bates, R. K. Olson, S. M. Davis, J. S. Flier, and M. G. Myers, Jr. 2000. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 275:40649-40657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
