Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity
- PMID: 15143187
- PMCID: PMC416404
- DOI: 10.1128/MCB.24.11.4955-4967.2004
Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity
Abstract
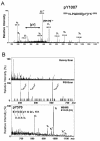
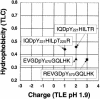
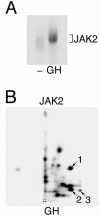
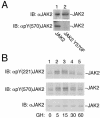
The tyrosine kinase JAK2 is a key signaling protein for at least 20 receptors in the cytokine/hematopoietin receptor superfamily and is a component of signaling by insulin receptor and several G-protein-coupled receptors. However, there is only limited knowledge of the physical structure of JAK2 or which of the 49 tyrosines in JAK2 are autophosphorylated. In this study, mass spectrometry and two-dimensional peptide mapping were used to determine that tyrosines 221, 570, and 1007 in JAK2 are autophosphorylated. Phosphorylation of tyrosine 570 is particularly robust. In response to growth hormone, JAK2 was rapidly and transiently phosphorylated at tyrosines 221 and 570, returning to basal levels by 60 min. Analysis of the sequences surrounding tyrosines 221 and 570 in JAK2 and tyrosines in other proteins that are phosphorylated in response to ligands that activate JAK2 suggests that the YXX[L/I/V] motif is one of the motifs recognized by JAK2. Experiments using JAK2 with tyrosines 221 and 570 mutated to phenylalanine suggest that tyrosines 221 and 570 in JAK2 may serve as regulatory sites in JAK2, with phosphorylation of tyrosine 221 increasing kinase activity and phosphorylation of tyrosine 570 decreasing kinase activity and thereby contributing to rapid termination of ligand activation of JAK2.
Figures





Similar articles
-
Tyrosines 868, 966, and 972 in the kinase domain of JAK2 are autophosphorylated and required for maximal JAK2 kinase activity.Mol Endocrinol. 2010 May;24(5):1062-76. doi: 10.1210/me.2009-0355. Epub 2010 Mar 19. Mol Endocrinol. 2010. PMID: 20304997 Free PMC article.
-
Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor.Mol Cell Biol. 2006 Jun;26(11):4052-62. doi: 10.1128/MCB.01591-05. Mol Cell Biol. 2006. PMID: 16705159 Free PMC article.
-
Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone.Nature. 1997 Nov 6;390(6655):91-6. doi: 10.1038/36369. Nature. 1997. PMID: 9363897
-
Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta.Mol Cell Biol. 2004 May;24(10):4557-70. doi: 10.1128/MCB.24.10.4557-4570.2004. Mol Cell Biol. 2004. PMID: 15121872 Free PMC article.
-
The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: its role in cell growth and disease.Philos Trans R Soc Lond B Biol Sci. 1998 Apr 29;353(1368):583-605. doi: 10.1098/rstb.1998.0228. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9602534 Free PMC article. Review.
Cited by
-
Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F.Nat Struct Mol Biol. 2012 Aug;19(8):754-9. doi: 10.1038/nsmb.2348. Epub 2012 Jul 22. Nat Struct Mol Biol. 2012. PMID: 22820988 Free PMC article.
-
Receptor specific downregulation of cytokine signaling by autophosphorylation in the FERM domain of Jak2.EMBO J. 2006 Oct 18;25(20):4763-72. doi: 10.1038/sj.emboj.7601365. Epub 2006 Oct 5. EMBO J. 2006. PMID: 17024180 Free PMC article.
-
Regulation of Jak2 function by phosphorylation of Tyr317 and Tyr637 during cytokine signaling.Mol Cell Biol. 2009 Jun;29(12):3367-78. doi: 10.1128/MCB.00278-09. Epub 2009 Apr 13. Mol Cell Biol. 2009. PMID: 19364823 Free PMC article.
-
Analysis of Jak2 catalytic function by peptide microarrays: the role of the JH2 domain and V617F mutation.PLoS One. 2011 Apr 18;6(4):e18522. doi: 10.1371/journal.pone.0018522. PLoS One. 2011. PMID: 21533163 Free PMC article.
-
JAK Kinases in Health and Disease: An Update.Open Rheumatol J. 2012;6:232-44. doi: 10.2174/1874312901206010232. Epub 2012 Sep 7. Open Rheumatol J. 2012. PMID: 23028408 Free PMC article.
References
-
- Ali, S., and S. Ali. 1998. Prolactin receptor regulates Stat5 tyrosine phosphorylation and nuclear translocation by two separate pathways. J. Biol. Chem. 273:7709-7716. - PubMed
-
- Arai, A., E. Kanda, Y. Nosaka, N. Miyasaka, and O. Miura. 2001. CrkL is recruited through its SH2 domain to the erythropoietin receptor and plays a role in Lyn-mediated receptor signaling. J. Biol. Chem. 276:33282-33290. - PubMed
-
- Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. - PubMed
-
- Argetsinger, L. S., and C. Carter-Su. 1996. Mechanism of signaling by growth hormone receptor. Physiol. Rev. 76:1089-1107. - PubMed
-
- Banks, A. S., S. M. Davis, S. H. Bates, and M. G. Myers, Jr. 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275:14563-14572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
