Characterization of a UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase with an unusual lectin domain from the platyhelminth parasite Echinococcus granulosus
- PMID: 15142032
- PMCID: PMC1133806
- DOI: 10.1042/BJ20031877
Characterization of a UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase with an unusual lectin domain from the platyhelminth parasite Echinococcus granulosus
Abstract
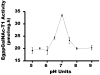
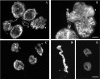
As part of a general project aimed at elucidating the initiation of mucin-type O-glycosylation in helminth parasites, we have characterized a novel ppGalNAc-T (UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase) from the cestode Echinococcus granulosus (Eg-ppGalNAc-T1). A full-length cDNA was isolated from a library of the tissue-dwelling larval stage of the parasite, and found to code for a 654-amino-acid protein containing all the structural features of ppGalNAc-Ts. Functional characterization of a recombinant protein lacking the transmembrane domain showed maximal activity at 28 degrees C, in the range 6.5-7.5 pH units and in the presence of Cu2+. In addition, it transferred GalNAc to a broad range of substrate peptides, derived from human mucins and O-glycosylated parasite proteins, including acceptors containing only serine or only threonine residues. Interestingly, the C-terminal region of Eg-ppGalNAc-T1 bears a highly unusual lectin domain, considerably longer than the one from other members of the family, and including only one of the three ricin B repeats generally present in ppGalNAc-Ts. Furthermore, a search for conserved domains within the protein C-terminus identified a fragment showing similarity to a recently defined domain, specialized in the binding of organic phosphates (CYTH). The role of the lectin domain in the determination of the substrate specificity of these enzymes suggests that Eg-ppGalNAc-T1 would be involved in the glycosylation of a special type of substrate. Analysis of the tissue distribution by in situ hybridization and immunohistochemistry revealed that this transferase is expressed in the hydatid cyst wall and the subtegumental region of larval worms. Therefore it could participate in the biosynthesis of O-glycosylated parasite proteins exposed at the interface between E. granulosus and its hosts.
Figures



Similar articles
-
cDNA cloning and expression of UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase T1 from Toxoplasma gondii.Mol Biochem Parasitol. 2003 Oct;131(2):93-107. doi: 10.1016/s0166-6851(03)00196-8. Mol Biochem Parasitol. 2003. PMID: 14511808
-
The lectin domain of the polypeptide GalNAc transferase family of glycosyltransferases (ppGalNAc Ts) acts as a switch directing glycopeptide substrate glycosylation in an N- or C-terminal direction, further controlling mucin type O-glycosylation.J Biol Chem. 2013 Jul 5;288(27):19900-14. doi: 10.1074/jbc.M113.477877. Epub 2013 May 20. J Biol Chem. 2013. PMID: 23689369 Free PMC article.
-
Cloning, expression and properties of porcine trachea UDP-galnac: polypeptide N-acetylgalactosaminyl transferase.Mol Cell Biochem. 2004 Nov;266(1-2):117-26. doi: 10.1023/b:mcbi.0000049148.73497.01. Mol Cell Biochem. 2004. PMID: 15646032
-
Polypeptide N-acetylgalactosaminyltransferase-Associated Phenotypes in Mammals.Molecules. 2021 Sep 10;26(18):5504. doi: 10.3390/molecules26185504. Molecules. 2021. PMID: 34576978 Free PMC article. Review.
-
Structure-based evolutionary relationship of glycosyltransferases: a case study of vertebrate β1,4-galactosyltransferase, invertebrate β1,4-N-acetylgalactosaminyltransferase and α-polypeptidyl-N-acetylgalactosaminyltransferase.Curr Opin Struct Biol. 2010 Oct;20(5):536-42. doi: 10.1016/j.sbi.2010.07.004. Epub 2010 Aug 11. Curr Opin Struct Biol. 2010. PMID: 20705453 Free PMC article. Review.
Cited by
-
UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase from the snail Biomphalaria glabrata - structural reflections.Glycoconj J. 2020 Feb;37(1):15-25. doi: 10.1007/s10719-019-09886-y. Epub 2019 Aug 8. Glycoconj J. 2020. PMID: 31396754 Free PMC article.
-
Conservation of peptide acceptor preferences between Drosophila and mammalian polypeptide-GalNAc transferase ortholog pairs.Glycobiology. 2008 Nov;18(11):861-70. doi: 10.1093/glycob/cwn073. Epub 2008 Jul 31. Glycobiology. 2008. PMID: 18669915 Free PMC article.
-
Glycopeptide-preferring polypeptide GalNAc transferase 10 (ppGalNAc T10), involved in mucin-type O-glycosylation, has a unique GalNAc-O-Ser/Thr-binding site in its catalytic domain not found in ppGalNAc T1 or T2.J Biol Chem. 2009 Jul 24;284(30):20387-97. doi: 10.1074/jbc.M109.017236. Epub 2009 May 21. J Biol Chem. 2009. PMID: 19460755 Free PMC article.
-
Biological and biochemical properties of two Xenopus laevis N-acetylgalactosaminyltransferases with contrasting roles in embryogenesis.Comp Biochem Physiol B Biochem Mol Biol. 2015 Feb;180:40-7. doi: 10.1016/j.cbpb.2014.10.003. Epub 2014 Oct 23. Comp Biochem Physiol B Biochem Mol Biol. 2015. PMID: 25447273 Free PMC article.
-
A transcriptomic analysis of Echinococcus granulosus larval stages: implications for parasite biology and host adaptation.PLoS Negl Trop Dis. 2012;6(11):e1897. doi: 10.1371/journal.pntd.0001897. Epub 2012 Nov 29. PLoS Negl Trop Dis. 2012. PMID: 23209850 Free PMC article.
References
-
- Thompson R. C. A. Biology and systematics of Echinococcus. In: Lymbery A. J., Thompson R. C. A., editors. Biology and Systematics of Echinococcus. Wallingford: CAB International; 1995. pp. 1–50.
-
- Theodoropoulos G., Hicks S., Corfield A., Miller B., Carrington S. The role of mucins in host–parasite interactions: part II – helminth parasites. Trends Parasitol. 2001;17:130–135. - PubMed
-
- Loukas A., Hintz M., Linder D., Mullin N., Parkinson J., Tetteh K., Maizels R. A family of secreted mucins from the parasitic nematode Toxocara canis bears diverse mucin domains but shares similar flanking six-cysteine repeat motifs. J. Biol. Chem. 2000;275:39600–39607. - PubMed
-
- Hülsmeier A., Gehrig P., Geyer R., Sack R., Gottstein B., Deplazes P., Köhler P. A major Echinococcus multilocularis antigen is a mucin-type glycoprotein. J. Biol. Chem. 2002;277:5742–5748. - PubMed
-
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989;52:257–331. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

