Functions of yeast helicase Ssl2p that are essential for viability are also involved in protection from the toxicity of adriamycin
- PMID: 15141027
- PMCID: PMC419470
- DOI: 10.1093/nar/gkh582
Functions of yeast helicase Ssl2p that are essential for viability are also involved in protection from the toxicity of adriamycin
Abstract
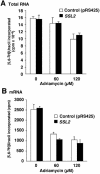
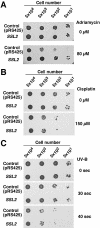
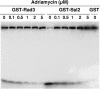
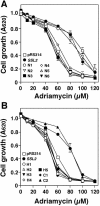
We have found that, in the yeast Saccharomyces cerevisiae, overexpression of the DNA helicase Ssl2p confers resistance to adriamycin. Ssl2p is involved, as a subunit of the basic transcription factor TFIIH, in the initiation of transcription and in nucleotide-excision repair (NER), and this helicase is essential for the survival of yeast cells. An examination of the relationship between the known functions of Ssl2p and adriamycin resistance indicated that overexpression of Ssl2p caused little or no increase in the rate of RNA synthesis and in NER. The absence of any involvement of NER in adriamycin resistance was supported by the finding that yeast cells that overexpressed the mutant form of Ssl2p that lacked the carboxy-terminal region, which is necessary for NER, remained resistant to adriamycin. When we examined the effects of overexpression in yeast of other mutant forms of Ssl2p with various deletions, we found that, of the 843 amino acids of Ssl2p, the entire amino acid sequence from position 81 to position 750 was necessary for adriamycin resistance. This region is identical to the region of Ssl2p that is necessary for the survival of yeast cells. Although this region contains helicase motifs, the overexpression of other yeast helicases, such as Rad3 and Sgs1, had little or no effect on adriamycin resistance, indicating that a mere increase in the intracellular level of helicases does not result in adriamycin resistance. Our results suggest that the functions of Ssl2p that are essential for yeast survival are also required for protection against adriamycin toxicity.
Figures



Similar articles
-
Overexpression of Ssl2p confers resistance to adriamycin and actinomycin D in Saccharomyces cerevisiae.Biochem Biophys Res Commun. 2004 Feb 13;314(3):844-8. doi: 10.1016/j.bbrc.2003.12.160. Biochem Biophys Res Commun. 2004. PMID: 14741713
-
Posttranslational inhibition of Ty1 retrotransposition by nucleotide excision repair/transcription factor TFIIH subunits Ssl2p and Rad3p.Genetics. 1998 Apr;148(4):1743-61. doi: 10.1093/genetics/148.4.1743. Genetics. 1998. PMID: 9560391 Free PMC article.
-
Transcription factor b (TFIIH) is required during nucleotide-excision repair in yeast.Nature. 1994 Mar 3;368(6466):74-6. doi: 10.1038/368074a0. Nature. 1994. PMID: 8107888
-
[Functional analysis of yeast homologue gene associated with human DNA helicase causative syndromes].Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2002;(120):53-74. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2002. PMID: 12638184 Review. Japanese.
-
The Rad3 protein from Saccharomyces cerevisiae: a DNA and DNA:RNA helicase with putative RNA helicase activity.Mol Microbiol. 1993 Mar;7(6):831-5. doi: 10.1111/j.1365-2958.1993.tb01173.x. Mol Microbiol. 1993. PMID: 8387143 Review.
Cited by
-
Host Chromatin Regulators Required for Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Activity in Saccharomyces cerevisiae Model.Infect Immun. 2021 Jul 15;89(8):e0003621. doi: 10.1128/IAI.00036-21. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 33941581 Free PMC article.
-
The protein transportation pathway from Golgi to vacuoles via endosomes plays a role in enhancement of methylmercury toxicity.Sci Rep. 2014 Jul 30;4:5888. doi: 10.1038/srep05888. Sci Rep. 2014. PMID: 25074250 Free PMC article.
-
High-Copy Overexpression Screening Reveals PDR5 as the Main Doxorubicin Resistance Gene in Yeast.PLoS One. 2015 Dec 21;10(12):e0145108. doi: 10.1371/journal.pone.0145108. eCollection 2015. PLoS One. 2015. PMID: 26690737 Free PMC article.
-
Comparative genome-wide screening identifies a conserved doxorubicin repair network that is diploid specific in Saccharomyces cerevisiae.PLoS One. 2009 Jun 8;4(6):e5830. doi: 10.1371/journal.pone.0005830. PLoS One. 2009. PMID: 19503795 Free PMC article.
References
-
- Benjamin R.S., Wiernik,P.H. and Bachur,N.R. (1974) Adriamycin chemotherapy—efficacy, safety and pharmacologic basis of an intermittent single high-dosage schedule. Cancer, 33, 19–27. - PubMed
-
- Gewirtz D.A. (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol., 57, 727–741. - PubMed
-
- Ueda K., Clark,D.P., Chen,C.J., Roninson,I.B., Gottesman,M.M. and Pastan,I. (1987) The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J. Biol. Chem., 262, 505–508. - PubMed
-
- Lincke C.R., van der Bliek,A.M., Schuurhuis,G.J., van der Velde-Koerts,T., Smit,J.J. and Borst,P. (1990) Multidrug resistance phenotype of human BRO melanoma cells transfected with a wild-type human mdr1 complementary DNA. Cancer Res., 50, 1779–1785. - PubMed
-
- Cole S.P., Bhardwaj,G., Gerlach,J.H., Mackie,J.E., Grant,C.E., Almquist,K.C., Stewart,A.J., Kurz,E.U., Duncan,A.M. and Deeley,R.G. (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science, 258, 1650–1654. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

