Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice
- PMID: 15128857
- PMCID: PMC6729445
- DOI: 10.1523/JNEUROSCI.2245-02.2004
Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice
Abstract
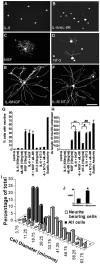
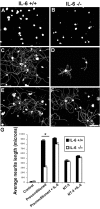
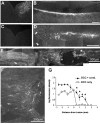
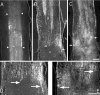
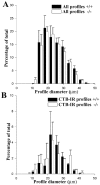
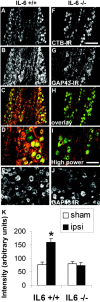
Regeneration of injured adult sensory neurons within the CNS is essentially abortive, attributable in part to lesion-induced or revealed inhibitors such as the chondroitin sulfate proteoglycans and the myelin inhibitors (Nogo-A, MAG, and OMgp). Much of this inhibition may be overcome by boosting the growth status of sensory neurons by delivering a conditioning lesion to their peripheral branches. Here, we identify a key role for the lesion-induced cytokine interleukin-6 (IL-6) in mediating conditioning lesion-induced enhanced regeneration of injured dorsal column afferents. In adult mice, conditioning injury to the sciatic nerve 1 week before bilateral dorsal column crush resulted in regeneration of dorsal column axons up to and beyond the injury site into host CNS tissue. This enhanced growth state was accompanied by an increase in the expression of the growth-associated protein GAP43 in preinjured but not intact dorsal root ganglia (DRGs). Preconditioning injury of the sciatic nerve in IL-6 -/- mice resulted in the total failure in regeneration of dorsal column axons consequent on the lack of GAP43 upregulation after a preconditioning injury. DRGs cell counts and cholera toxin beta subunit labeling revealed that impaired regeneration in knock-out mice was unrelated to cell loss or a deficit in tracer transport. In vitro, exogenous IL-6 boosted sensory neuron growth status as evidenced by enhanced neurite extension. This effect required NGF or NT-3 but not soluble IL-6 receptor as cofactors. Evidence of conditioning lesion-enhanced growth status of DRGs cells can also be observed in vitro as an earlier and enhanced rate of neurite extension; this phenomenon fails in IL-6 -/- mice preinjured 7 d in vivo. We suggest that injury-induced IL-6 upregulation is required to promote regeneration within the CNS. Our results indicate that this is achieved through a boosted growth state of dorsal column projecting sensory neurons.
Figures

Similar articles
-
Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation.J Neurosci. 2005 Feb 16;25(7):1645-53. doi: 10.1523/JNEUROSCI.3269-04.2005. J Neurosci. 2005. PMID: 15716400 Free PMC article.
-
Intraneural Injection of ATP Stimulates Regeneration of Primary Sensory Axons in the Spinal Cord.J Neurosci. 2018 Feb 7;38(6):1351-1365. doi: 10.1523/JNEUROSCI.1660-17.2017. Epub 2017 Dec 26. J Neurosci. 2018. PMID: 29279307 Free PMC article.
-
Complement protein C1q modulates neurite outgrowth in vitro and spinal cord axon regeneration in vivo.J Neurosci. 2015 Mar 11;35(10):4332-49. doi: 10.1523/JNEUROSCI.4473-12.2015. J Neurosci. 2015. PMID: 25762679 Free PMC article.
-
The Dorsal Column Lesion Model of Spinal Cord Injury and Its Use in Deciphering the Neuron-Intrinsic Injury Response.Dev Neurobiol. 2018 Oct;78(10):926-951. doi: 10.1002/dneu.22601. Epub 2018 May 11. Dev Neurobiol. 2018. PMID: 29717546 Free PMC article. Review.
-
Expression of Regeneration-Associated Proteins in Primary Sensory Neurons and Regenerating Axons After Nerve Injury-An Overview.Anat Rec (Hoboken). 2018 Oct;301(10):1618-1627. doi: 10.1002/ar.23843. Epub 2018 May 13. Anat Rec (Hoboken). 2018. PMID: 29740961 Review.
Cited by
-
The Conditioning Lesion Response in Dorsal Root Ganglion Neurons Is Inhibited in Oncomodulin Knock-Out Mice.eNeuro. 2022 Feb 24;9(1):ENEURO.0477-21.2022. doi: 10.1523/ENEURO.0477-21.2022. Print 2022 Jan-Feb. eNeuro. 2022. PMID: 35131866 Free PMC article.
-
Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke.J Clin Invest. 2008 Jul;118(7):2482-95. doi: 10.1172/JCI34363. J Clin Invest. 2008. PMID: 18596986 Free PMC article.
-
Peripheral nerve regeneration and NGF-dependent neurite outgrowth of adult sensory neurons converge on STAT3 phosphorylation downstream of neuropoietic cytokine receptor gp130.J Neurosci. 2014 Sep 24;34(39):13222-33. doi: 10.1523/JNEUROSCI.1209-13.2014. J Neurosci. 2014. PMID: 25253866 Free PMC article.
-
Oncostatin M reduces lesion size and promotes functional recovery and neurite outgrowth after spinal cord injury.Mol Neurobiol. 2014 Dec;50(3):1142-51. doi: 10.1007/s12035-014-8795-5. Epub 2014 Jul 5. Mol Neurobiol. 2014. PMID: 24996996
-
Waking up the sleepers: shared transcriptional pathways in axonal regeneration and neurogenesis.Cell Mol Life Sci. 2013 Mar;70(6):993-1007. doi: 10.1007/s00018-012-1099-x. Epub 2012 Aug 17. Cell Mol Life Sci. 2013. PMID: 22899311 Free PMC article. Review.
References
-
- Andersen LB, Schreyer DJ (1999) Constitutive expression of GAP-43 correlates with rapid, but not slow regrowth of injured dorsal root axons in the adult rat. Exp Neurol 155: 157–164. - PubMed
-
- Bjorklund A, Wiklund L, Descarries L (1981) Regeneration and plasticity of central serotonergic neurons: a review. J Physiol (Paris) 77: 247–255. - PubMed
-
- Bolin LM, Verity AN, Silver JE, Shooter EM, Abrams JS (1995) Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J Neurochem 64: 850–858. - PubMed
-
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci 4: 38–43. - PubMed
-
- Bourde O, Kiefer R, Toyka KV, Hartung HP (1996) Quantification of interleukin-6 mRNA in Wallerian degeneration by competitive reverse transcription polymerase chain reaction. J Neuroimmunol 69: 135–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
