Cellular effects of deoxynojirimycin analogues: inhibition of N-linked oligosaccharide processing and generation of free glucosylated oligosaccharides
- PMID: 15128289
- PMCID: PMC1133898
- DOI: 10.1042/BJ20031824
Cellular effects of deoxynojirimycin analogues: inhibition of N-linked oligosaccharide processing and generation of free glucosylated oligosaccharides
Abstract
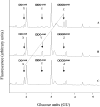
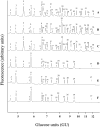
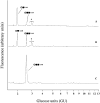
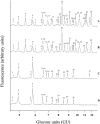
In the accompanying paper [Mellor, Neville, Harvey, Platt, Dwek and Butters (2004) Biochem. J. 381, 861-866] we treated HL60 cells with N-alk(en)yl-deoxynojirimycin (DNJ) compounds to inhibit glucosphingolipid (GSL) biosynthesis and identified a number of non-GSL-derived, small, free oligosaccharides (FOS) most likely produced due to inhibition of the oligosaccharide-processing enzymes a-glucosidases I and II. When HL60 cells were treated with concentrations of N-alk(en)ylated DNJ analogues that inhibited GSL biosynthesis completely, N-butyl- and N-nonyl-DNJ inhibited endoplasmic reticulum (ER) glucosidases I and II, but octadecyl-DNJ did not, probably due to the lack of ER lumen access for this novel, long-chain derivative. Glucosidase inhibition resulted in the appearance of free Glc1-3Man structures, which is evidence of Golgi glycoprotein endomannosidase processing of oligosaccharides with retained glucose residues. Additional large FOS was also detected in cells following a 16 h treatment with N-butyl- and N-nonyl-DNJ. When these FOS structures (>30, including >20 species not present in control cells) were characterized by enzyme digests and MALDI-TOF (matrix-assisted laser-desorption ionization-time-of-flight) MS, all were found to be polymannose-type oligosaccharides, of which the majority were glucosylated and had only one reducing terminal GlcNAc (N-acetylglucosamine) residue (FOS-GlcNAc1), demonstrating a cytosolic location. These results support the proposal that the increase in glucosylated FOS results from enzyme-mediated cytosolic cleavage of oligosaccharides from glycoproteins exported from the ER because of misfolding or excessive retention. Importantly, the present study characterizes the cellular properties of DNJs further and demonstrates that side-chain modifications allow selective inhibition of protein and lipid glycosylation pathways. This represents the most detailed characterization of the FOS structures arising from ER a-glucosidase inhibition to date.
Figures

Similar articles
-
Cellular effects of deoxynojirimycin analogues: uptake, retention and inhibition of glycosphingolipid biosynthesis.Biochem J. 2004 Aug 1;381(Pt 3):861-6. doi: 10.1042/BJ20031822. Biochem J. 2004. PMID: 15128268 Free PMC article.
-
Glucosylated free oligosaccharides are biomarkers of endoplasmic- reticulum alpha-glucosidase inhibition.Biochem J. 2008 Jan 15;409(2):571-80. doi: 10.1042/BJ20070748. Biochem J. 2008. PMID: 17868040
-
Characterization of endomannosidase inhibitors and evaluation of their effect on N-linked oligosaccharide processing during glycoprotein biosynthesis.J Biol Chem. 1993 May 5;268(13):9927-35. J Biol Chem. 1993. PMID: 8486671
-
Inhibitors of the biosynthesis and processing of N-linked oligosaccharides.CRC Crit Rev Biochem. 1984;16(1):21-49. doi: 10.3109/10409238409102805. CRC Crit Rev Biochem. 1984. PMID: 6232113 Review.
-
Manipulation of the biosynthesis of protein-modifying glycoconjugates by the use of specific inhibitors.Behring Inst Mitt. 1991 Jul;(89):198-208. Behring Inst Mitt. 1991. PMID: 1834053 Review.
Cited by
-
Man2C1, an alpha-mannosidase, is involved in the trimming of free oligosaccharides in the cytosol.Biochem J. 2006 Nov 15;400(1):33-41. doi: 10.1042/BJ20060945. Biochem J. 2006. PMID: 16848760 Free PMC article.
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2003-2004.Mass Spectrom Rev. 2009 Mar-Apr;28(2):273-361. doi: 10.1002/mas.20192. Mass Spectrom Rev. 2009. PMID: 18825656 Free PMC article. Review.
-
Cellular effects of deoxynojirimycin analogues: uptake, retention and inhibition of glycosphingolipid biosynthesis.Biochem J. 2004 Aug 1;381(Pt 3):861-6. doi: 10.1042/BJ20031822. Biochem J. 2004. PMID: 15128268 Free PMC article.
-
Strategy for Designing Selective Lysosomal Acid α-Glucosidase Inhibitors: Binding Orientation and Influence on Selectivity.Molecules. 2020 Jun 19;25(12):2843. doi: 10.3390/molecules25122843. Molecules. 2020. PMID: 32575625 Free PMC article.
-
Iminosugar antivirals: the therapeutic sweet spot.Biochem Soc Trans. 2017 Apr 15;45(2):571-582. doi: 10.1042/BST20160182. Biochem Soc Trans. 2017. PMID: 28408497 Free PMC article. Review.
References
-
- Butters T. D., van den Broek L., Fleet G. W. J., Krulle T. M., Wormald M. R., Dwek R. A., Platt F. M. Molecular requirements of imino sugars for the selective control of N-linked glycosylation and glycosphingolipid biosynthesis. Tetrahedron: Asymmetry. 2000;11:113–124.
-
- Platt F. M., Neises G. R., Dwek R. A., Butters T. D. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J. Biol. Chem. 1994;269:8362–8365. - PubMed
-
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. - PubMed
-
- Helenius A., Trombetta E. S., Herbert D. N., Simons J. F. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

