Acquisition of Hrs, an essential component of phagosomal maturation, is impaired by mycobacteria
- PMID: 15121875
- PMCID: PMC400451
- DOI: 10.1128/MCB.24.10.4593-4604.2004
Acquisition of Hrs, an essential component of phagosomal maturation, is impaired by mycobacteria
Abstract
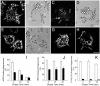
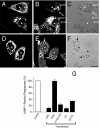
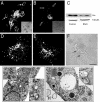
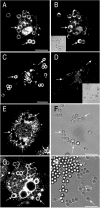
Pathogenic mycobacteria survive within macrophages by precluding the fusion of phagosomes with late endosomes or lysosomes. Because the molecular determinants of normal phagolysosome formation are poorly understood, the sites targeted by mycobacteria remain unidentified. We found that Hrs, an adaptor molecule involved in protein sorting, associates with phagosomes prior to their fusion with late endosomes or lysosomes. Recruitment of Hrs required the interaction of its FYVE domain with phagosomal phosphatidylinositol 3-phosphate, but two other attachment sites were additionally involved. Depletion of Hrs by use of small interfering RNA impaired phagosomal maturation, preventing the acquisition of lysobisphosphatidic acid and reducing luminal acidification. As a result, the maturation of phagosomes formed in Hrs-depleted cells was arrested at an early stage, characterized by the acquisition and retention of sorting endosomal markers. This phenotype is strikingly similar to that reported to occur in phagosomes of cells infected by mycobacteria. We therefore tested whether Hrs is recruited to phagosomes containing mycobacteria. Hrs associated readily with phagosomes containing inert particles but poorly with mycobacterial phagosomes. Moreover, Hrs was found more frequently in phagosomes containing avirulent Mycobacterium smegmatis than in phagosomes with the more virulent Mycobacterium marinum. These findings suggest that the inability to recruit Hrs contributes to the arrest of phagosomal maturation induced by pathogenic mycobacteria.
Figures


Similar articles
-
The myotubularin MTMR4 regulates phagosomal phosphatidylinositol 3-phosphate turnover and phagocytosis.J Biol Chem. 2019 Nov 8;294(45):16684-16697. doi: 10.1074/jbc.RA119.009133. Epub 2019 Sep 22. J Biol Chem. 2019. PMID: 31543504 Free PMC article.
-
Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest.J Cell Biol. 2001 Aug 6;154(3):631-44. doi: 10.1083/jcb.200106049. J Cell Biol. 2001. PMID: 11489920 Free PMC article.
-
Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase.Mol Cell Biol. 2003 Apr;23(7):2501-14. doi: 10.1128/MCB.23.7.2501-2514.2003. Mol Cell Biol. 2003. PMID: 12640132 Free PMC article.
-
Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking.Electrophoresis. 1997 Dec;18(14):2542-7. doi: 10.1002/elps.1150181409. Electrophoresis. 1997. PMID: 9527483 Review.
-
Phosphoinositides in phagolysosome and autophagosome biogenesis.Biochem Soc Symp. 2007;(74):141-8. doi: 10.1042/BSS0740141. Biochem Soc Symp. 2007. PMID: 17233587 Review.
Cited by
-
Macrophage-microbe interaction: lessons learned from the pathogen Mycobacterium tuberculosis.Semin Immunopathol. 2018 Nov;40(6):577-591. doi: 10.1007/s00281-018-0710-0. Epub 2018 Oct 10. Semin Immunopathol. 2018. PMID: 30306257 Review.
-
A Rab-centric perspective of bacterial pathogen-occupied vacuoles.Cell Host Microbe. 2013 Sep 11;14(3):256-68. doi: 10.1016/j.chom.2013.08.010. Cell Host Microbe. 2013. PMID: 24034612 Free PMC article. Review.
-
The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins.Nature. 2009 Mar 26;458(7237):445-52. doi: 10.1038/nature07961. Nature. 2009. PMID: 19325624 Review.
-
Monitoring Phosphoinositide Fluxes and Effectors During Leukocyte Chemotaxis and Phagocytosis.Front Cell Dev Biol. 2021 Feb 4;9:626136. doi: 10.3389/fcell.2021.626136. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33614656 Free PMC article. Review.
-
Mechanistic similarities in docking of the FYVE and PX domains to phosphatidylinositol 3-phosphate containing membranes.Prog Lipid Res. 2007 Nov;46(6):315-27. doi: 10.1016/j.plipres.2007.06.001. Epub 2007 Jul 13. Prog Lipid Res. 2007. PMID: 17707914 Free PMC article. Review.
References
-
- Adam, T. 2001. Exploitation of host factors for efficient infection by Shigella. Int. J. Med. Microbiol. 291:287-298. - PubMed
-
- Bean, A. J., R. Seifert, Y. A. Chen, R. Sacks, and R. H. Scheller. 1997. Hrs-2 is an ATPase implicated in calcium-regulated secretion. Nature 385:826-829. - PubMed
-
- Chin, L. S., M. C. Raynor, X. Wei, H. Q. Chen, and L. Li. 2001. Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 276:7069-7078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
