Comparison of the Rta/Orf50 transactivator proteins of gamma-2-herpesviruses
- PMID: 15113928
- PMCID: PMC400334
- DOI: 10.1128/jvi.78.10.5491-5499.2004
Comparison of the Rta/Orf50 transactivator proteins of gamma-2-herpesviruses
Abstract
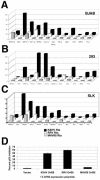
The viral immediate-early transactivator Rta/Orf50 is necessary and sufficient to initiate Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) reactivation from latently infected cells. Since Rta/Orf50 is conserved among all known gamma-2-herpesviruses, we investigated whether the murine gamma-68-herpesvirus (MHV-68) and rhesus monkey rhadinovirus (RRV) homologs can functionally substitute for KSHV Rta/Orf50. (i) Our comparison of 12 KSHV promoters showed that most responded to all three Rta/Orf50proteins, but three promoters (vGPCR, K8, and gB) responded only to the KSHV Rta/Orf50 transactivator. Overall, the activation of KSHV promoters was higher with KSHV Rta than with the RRV and MHV-68 Rta. (ii) Only the primate Rta/Orf50 homologs were able to interfere with human p53-depedent transcriptional activation. (iii) Transcriptional profiling showed that the KSHV Rta/Orf50 was more efficient than it's homologs in inducing KSHV lytic transcription from the latent state. These results suggest that the core functionality of Rta/Orf50 is conserved and independent of its host, but the human protein has evolved additional, human-specific capabilities.
Figures


Similar articles
-
The Rta/Orf50 transactivator proteins of the gamma-herpesviridae.Curr Top Microbiol Immunol. 2007;312:71-100. doi: 10.1007/978-3-540-34344-8_3. Curr Top Microbiol Immunol. 2007. PMID: 17089794 Review.
-
Kinetics of expression of rhesus monkey rhadinovirus (RRV) and identification and characterization of a polycistronic transcript encoding the RRV Orf50/Rta, RRV R8, and R8.1 genes.J Virol. 2002 Oct;76(19):9819-31. doi: 10.1128/jvi.76.19.9819-9831.2002. J Virol. 2002. PMID: 12208960 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50/Rta lytic switch protein functions as a tetramer.J Virol. 2007 Jun;81(11):5788-806. doi: 10.1128/JVI.00140-07. Epub 2007 Mar 28. J Virol. 2007. PMID: 17392367 Free PMC article.
-
Identification of Novel Kaposi's Sarcoma-Associated Herpesvirus Orf50 Transcripts: Discovery of New RTA Isoforms with Variable Transactivation Potential.J Virol. 2016 Dec 16;91(1):e01434-16. doi: 10.1128/JVI.01434-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795414 Free PMC article.
-
Rhesus monkey rhadinovirus: a model for the study of KSHV.Curr Top Microbiol Immunol. 2007;312:43-69. doi: 10.1007/978-3-540-34344-8_2. Curr Top Microbiol Immunol. 2007. PMID: 17089793 Review.
Cited by
-
Binding of RBP-Jkappa (CSL) protein to the promoter of the Kaposi's sarcoma-associated herpesvirus ORF47 (gL) gene is a critical but not sufficient determinant of transactivation by ORF50 protein.Virology. 2010 Mar 1;398(1):38-48. doi: 10.1016/j.virol.2009.11.022. Epub 2009 Dec 16. Virology. 2010. PMID: 20006367 Free PMC article.
-
Molecular biology of KSHV in relation to AIDS-associated oncogenesis.Cancer Treat Res. 2007;133:69-127. doi: 10.1007/978-0-387-46816-7_3. Cancer Treat Res. 2007. PMID: 17672038 Free PMC article. Review.
-
Control of Rta expression critically determines transcription of viral and cellular genes following gammaherpesvirus infection.J Gen Virol. 2007 Jun;88(Pt 6):1689-1697. doi: 10.1099/vir.0.82548-0. J Gen Virol. 2007. PMID: 17485528 Free PMC article.
-
KSHV RTA antagonizes SMC5/6 complex-induced viral chromatin compaction by hijacking the ubiquitin-proteasome system.PLoS Pathog. 2022 Aug 1;18(8):e1010744. doi: 10.1371/journal.ppat.1010744. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35914008 Free PMC article.
-
KSHV RTA Induces Degradation of the Host Transcription Repressor ID2 To Promote the Viral Lytic Cycle.J Virol. 2022 Jun 22;96(12):e0010122. doi: 10.1128/jvi.00101-22. Epub 2022 May 23. J Virol. 2022. PMID: 35604218 Free PMC article.
References
-
- Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. - PMC - PubMed
-
- Chang, J., and D. Ganem. 2000. On the control of late gene expression in Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8). J. Gen. Virol. 81:2039-2047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

