Regional differences in viral growth and central nervous system injury correlate with apoptosis
- PMID: 15113925
- PMCID: PMC400348
- DOI: 10.1128/jvi.78.10.5466-5475.2004
Regional differences in viral growth and central nervous system injury correlate with apoptosis
Abstract
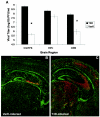
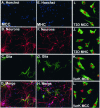
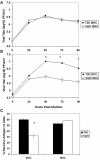
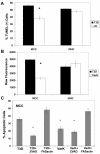
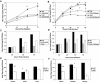
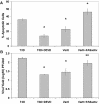
Infection of neonatal mice with reovirus T3 Dearing (T3D), the prototypic neurotropic reovirus, causes fatal encephalitis associated with neuronal injury and virus-induced apoptosis throughout the brain. T3D variant K (VarK) is an antigenic variant that has a nearly 1 million-fold reduction in neurovirulence following intracerebral (i.c.) inoculation compared to T3D and a restricted pattern of central nervous system injury with damage limited to the hippocampus, sparing other brain regions. We wished to determine whether the restricted pattern of VarK-induced injury was due to a reduced capacity to replicate in or injure cortical, as opposed to hippocampal, tissue. We found that following i.c. inoculation, VarK grew to similar titers as T3D in the hippocampus but had significantly lower titers in the cortex. Both viruses grew to identical titers and infected the same percentage of cells in mouse primary hippocampal cultures (MHC). In mouse primary cortical cultures (MCC) both the number of infected cells and the viral yield per infected cell were significantly lower for VarK than T3D. VarK-induced apoptosis was limited to the hippocampus in vivo, and in vitro both viruses induced apoptosis equally in MHC but VarK induced significantly less apoptosis than T3D in MCC. Growth of T3D in MCC was reduced to levels comparable to those of VarK following treatment of MCC with caspase inhibitors. Conversely, induction of apoptosis in VarK-infected MCC with fatty acid synthase-activating antibody significantly enhanced viral yield. These results suggest that the decreased neurovirulence of VarK may be due to its failure to efficiently induce apoptosis in cortical neurons.
Figures


Similar articles
-
The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis.J Virol. 2011 Apr;85(8):3858-71. doi: 10.1128/JVI.01958-10. Epub 2011 Feb 9. J Virol. 2011. PMID: 21307199 Free PMC article.
-
Brain- and intestine-specific variants of reovirus serotype 3 strain dearing are selected during chronic infection of severe combined immunodeficient mice.J Virol. 1995 Jun;69(6):3933-7. doi: 10.1128/JVI.69.6.3933-3937.1995. J Virol. 1995. PMID: 7745749 Free PMC article.
-
Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis.J Virol. 1997 Mar;71(3):2100-6. doi: 10.1128/JVI.71.3.2100-2106.1997. J Virol. 1997. PMID: 9032342 Free PMC article.
-
Apoptosis and the cytopathic effects of reovirus.Curr Top Microbiol Immunol. 1998;233(Pt 2):23-49. doi: 10.1007/978-3-642-72095-6_2. Curr Top Microbiol Immunol. 1998. PMID: 9599930 Review. No abstract available.
-
Mechanisms of reovirus-induced cell death and tissue injury: role of apoptosis and virus-induced perturbation of host-cell signaling and transcription factor activation.Viral Immunol. 2005;18(1):89-115. doi: 10.1089/vim.2005.18.89. Viral Immunol. 2005. PMID: 15802955 Free PMC article. Review.
Cited by
-
Cell Killing by Reovirus: Mechanisms and Consequences.Curr Top Microbiol Immunol. 2023;442:133-153. doi: 10.1007/82_2020_225. Curr Top Microbiol Immunol. 2023. PMID: 32986138 Review.
-
The Anti-inflammatory Compound Candesartan Cilexetil Improves Neurological Outcomes in a Mouse Model of Neonatal Hypoxia.Front Immunol. 2019 Jul 24;10:1752. doi: 10.3389/fimmu.2019.01752. eCollection 2019. Front Immunol. 2019. PMID: 31396238 Free PMC article.
-
Daxx upregulation within the cytoplasm of reovirus-infected cells is mediated by interferon and contributes to apoptosis.J Virol. 2013 Mar;87(6):3447-60. doi: 10.1128/JVI.02324-12. Epub 2013 Jan 9. J Virol. 2013. PMID: 23302889 Free PMC article.
-
Reovirus activates a caspase-independent cell death pathway.mBio. 2013 May 14;4(3):e00178-13. doi: 10.1128/mBio.00178-13. mBio. 2013. PMID: 23674612 Free PMC article.
-
Mechanisms of apoptosis during reovirus infection.Curr Top Microbiol Immunol. 2005;289:1-24. doi: 10.1007/3-540-27320-4_1. Curr Top Microbiol Immunol. 2005. PMID: 15791949 Free PMC article. Review.
References
-
- Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. - PubMed
-
- Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

