The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1
- PMID: 15113878
- PMCID: PMC400377
- DOI: 10.1128/jvi.78.10.4983-4992.2004
The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1
Abstract
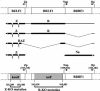
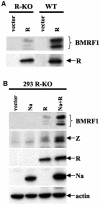
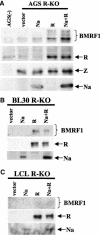
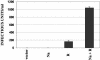
The switch from the latent to the lytic form of Epstein-Barr virus (EBV) infection is mediated by expression of the viral immediate-early (IE) proteins, BZLF1 (Z) and BRLF1 (R). An EBV early protein, BRRF1 (Na), is encoded by the opposite strand of the BRLF1 intron, but the function of this nuclear protein in the viral life cycle is unknown. Here we demonstrate that Na enhances the R-mediated induction of lytic EBV infection in 293 cells latently infected with a recombinant EBV (R-KO) defective for the expression of both R and Na. Na also enhances R-induced lytic infections in a gastric carcinoma line (AGS) carrying the R-KO virus, although it has no effect in a Burkitt lymphoma line (BL-30) stably infected with the same mutant virus. We show that Na is a transcription factor that increases the ability of R to activate Z expression from the R-KO viral genome in 293 cells and that Na by itself activates the Z promoter (Zp) in EBV-negative cells. Na activation of Zp requires a CRE motif (ZII), and a consensus CRE motif is sufficient to transfer Na responsiveness to the heterologous E1b promoter. Furthermore, we show that Na enhances the transactivator function of a Gal4-c-Jun fusion protein but does not increase the transactivator function of other transcription factors (including ATF-1, ATF-2, and CREB) known to bind CRE motifs. Na expression in cells results in increased levels of a hyperphosphorylated form of c-Jun, suggesting a mechanism by which Na activates c-Jun. Our results indicate that Na is a transcription factor that activates the EBV Zp IE promoter through its effects on c-Jun and suggest that Na cooperates with BRLF1 to induce the lytic form of EBV infection in certain cell types.
Figures




Similar articles
-
Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein BRLF1 and is required for lytic viral gene expression.J Virol. 2004 Apr;78(8):4197-206. doi: 10.1128/jvi.78.8.4197-4206.2004. J Virol. 2004. PMID: 15047835 Free PMC article.
-
Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases.J Virol. 2000 Feb;74(3):1224-33. doi: 10.1128/jvi.74.3.1224-1233.2000. J Virol. 2000. PMID: 10627532 Free PMC article.
-
Amino acid substitutions reveal distinct functions of serine 186 of the ZEBRA protein in activation of early lytic cycle genes and synergy with the Epstein-Barr virus R transactivator.J Virol. 1999 Jun;73(6):4543-51. doi: 10.1128/JVI.73.6.4543-4551.1999. J Virol. 1999. PMID: 10233912 Free PMC article.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
-
Molecular Basis of Epstein-Barr Virus Latency Establishment and Lytic Reactivation.Viruses. 2021 Nov 23;13(12):2344. doi: 10.3390/v13122344. Viruses. 2021. PMID: 34960613 Free PMC article. Review.
Cited by
-
Identification and characterization of the Orf49 protein of Kaposi's sarcoma-associated herpesvirus.J Virol. 2006 Mar;80(6):3062-70. doi: 10.1128/JVI.80.6.3062-3070.2006. J Virol. 2006. PMID: 16501115 Free PMC article.
-
Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model.J Virol. 2005 Nov;79(22):13993-4003. doi: 10.1128/JVI.79.22.13993-14003.2005. J Virol. 2005. PMID: 16254335 Free PMC article.
-
Reactivation of Epstein-Barr Virus by HIF-1α Requires p53.J Virol. 2020 Aug 31;94(18):e00722-20. doi: 10.1128/JVI.00722-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641480 Free PMC article.
-
Epstein-Barr virus WZhet DNA can induce lytic replication in epithelial cells in vitro, although WZhet is not detectable in many human tissues in vivo.Intervirology. 2009;52(1):8-16. doi: 10.1159/000210833. Epub 2009 Apr 7. Intervirology. 2009. PMID: 19349713 Free PMC article.
-
Viral genome methylation differentially affects the ability of BZLF1 versus BRLF1 to activate Epstein-Barr virus lytic gene expression and viral replication.J Virol. 2013 Jan;87(2):935-50. doi: 10.1128/JVI.01790-12. Epub 2012 Nov 7. J Virol. 2013. PMID: 23135711 Free PMC article.
References
-
- Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. - PMC - PubMed
-
- Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. - PubMed
-
- Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

