Local and systemic antibody responses in mice immunized intranasally with native and detergent-extracted outer membrane vesicles from Neisseria meningitidis
- PMID: 15102760
- PMCID: PMC387915
- DOI: 10.1128/IAI.72.5.2528-2537.2004
Local and systemic antibody responses in mice immunized intranasally with native and detergent-extracted outer membrane vesicles from Neisseria meningitidis
Abstract
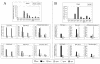
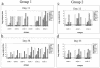
The mouse humoral immune response toward native or detergent-extracted outer membrane vesicles (NOMVs and DOMVs, respectively) from Neisseria meningitidis was determined after intranasal immunization. Both preparations elicited high frequencies of NOMV-specific antibody-forming cells (AFCs) locally in the nasal associated lymphoid tissue (NALT) after three or four weekly doses. The diffuse NALT (D-NALT) contained ca. 10-fold more NOMV-specific AFCs than those observed in the mediastinal lymph node, spleen, and bone marrow. AFCs observed in the D-NALT were primarily immunoglobulin A positive (IgA(+)) and were maintained for at least 1 month. In contrast, the organized NALT (O-NALT) contained low numbers of AFCs, and the response was relatively short-lived. In other lymphoid tissues, AFCs producing various IgG subclasses and IgM were present with IgG2b-producing AFCs being dominant or codominant with IgA or IgG2a. In serum and in all of the tissues examined, with the exception of the NALT, NOMVs clearly induced a stronger antibody response and a broader range of antibody isotypes than DOMVs. The development of NOMV-specific AFCs in spleen and bone marrow after intranasal immunization was slow compared to intravenous immunization but, once established, the intranasally elicited responses increased steadily for at least 75 days. NOMV-specific antibodies induced via several routes of immunization had high bactericidal activities in serum. Our results indicated that intranasally administered OMVs induced strong local and systemic antibody responses in mice that were relatively long-lived.
Figures




Similar articles
-
Immunogenicity of intranasally administered meningococcal native outer membrane vesicles in mice.Infect Immun. 1999 Jan;67(1):113-9. doi: 10.1128/IAI.67.1.113-119.1999. Infect Immun. 1999. PMID: 9864204 Free PMC article.
-
A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines.Clin Vaccine Immunol. 2011 May;18(5):736-42. doi: 10.1128/CVI.00542-10. Epub 2011 Mar 2. Clin Vaccine Immunol. 2011. PMID: 21367981 Free PMC article.
-
Meningococcal outer membrane vesicle vaccine given intranasally can induce immunological memory and booster responses without evidence of tolerance.Infect Immun. 2001 Aug;69(8):5010-5. doi: 10.1128/IAI.69.8.5010-5015.2001. Infect Immun. 2001. PMID: 11447180 Free PMC article.
-
Kinetics of mouse antibody and lymphocyte responses during intranasal vaccination with a lipooligosaccharide-based conjugate vaccine.Immunol Lett. 2006 Nov 15;107(2):131-9. doi: 10.1016/j.imlet.2006.08.005. Epub 2006 Sep 12. Immunol Lett. 2006. PMID: 17030407
-
Immunogenicity and safety testing of a group B intranasal meningococcal native outer membrane vesicle vaccine.Infect Immun. 2002 Feb;70(2):702-7. doi: 10.1128/IAI.70.2.702-707.2002. Infect Immun. 2002. PMID: 11796602 Free PMC article. Clinical Trial.
Cited by
-
Neisseria meningitidis Factor H Binding Protein Surface Exposure on Salmonella Typhimurium GMMA Is Critical to Induce an Effective Immune Response against Both Diseases.Pathogens. 2021 Jun 9;10(6):726. doi: 10.3390/pathogens10060726. Pathogens. 2021. PMID: 34207575 Free PMC article.
-
Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice.Infect Immun. 2008 Oct;76(10):4554-63. doi: 10.1128/IAI.00532-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678672 Free PMC article.
-
Immune modulation by bacterial outer membrane vesicles.Nat Rev Immunol. 2015 Jun;15(6):375-87. doi: 10.1038/nri3837. Epub 2015 May 15. Nat Rev Immunol. 2015. PMID: 25976515 Review.
-
Protection against neonatal enteric colibacillosis employing E. Coli-derived outer membrane vesicles in formulation and without vitamin D3.BMC Res Notes. 2018 May 16;11(1):302. doi: 10.1186/s13104-018-3442-2. BMC Res Notes. 2018. PMID: 29769118 Free PMC article.
-
Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination.Infect Immun. 2005 Jul;73(7):4070-80. doi: 10.1128/IAI.73.7.4070-4080.2005. Infect Immun. 2005. PMID: 15972495 Free PMC article.
References
-
- Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. - PubMed
-
- Alving, C. R. 1993. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology 187:430-446. - PubMed
-
- Andersen, S. R., G. Bjune, J. Lyngby, K. Bryn, and E. Jantzen. 1995. Short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis for use in the production of outer membrane vesicle vaccines. Microb. Pathog. 19:159-168. - PubMed
-
- Andersen, S. R., G. Bjune, E. A. Høiby, T. E. Michaelsen, A. Aase, U. Rye, and E. Jantzen. 1997. Outer membrane vesicle vaccines made from short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis: effect of the carbohydrate chain length on the immune response. Vaccine 15:1225-1234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

