Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation
- PMID: 15086400
- PMCID: PMC1809031
- DOI: 10.1111/j.1365-2249.2004.02426.x
Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation
Abstract
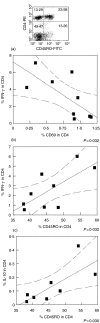
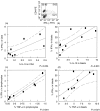
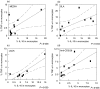
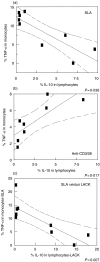
Regulation of the immune response directed against Leishmania is critical for the establishment of effective control of the disease. It is likely that some types of immune responses directed against Leishmania can lead to more severe clinical forms of leishmaniasis causing a poor control of the pathogen and/or pathology, while others lead to resolution of the infection with little pathology as in cutaneous leishmaniasis. To gain a better understanding of the possible role that subpopulations of T cells, and their associated cytokines have on disease progression and/or protective immune responses to L. braziliensis infection, a detailed study of the frequency of activated and memory T cells, as well as antigen specific, cytokine producing T cells was carried out. Following the determination of cytokine producing mononuclear cell populations in response to total Leishmania antigen (SLA), and to the recombinant antigen LACK, correlation analysis were performed between specific cytokine producing populations to identify models for cellular mechanisms of immunoregulation in human cutaneous leishmaniasis. These studies have shown: (1) a positive correlation between ex vivo CD45RO frequencies and antigen specific cytokine (IFN-gamma or IL-10) producing cells; (2) a negative correlation between ex vivo CD69 expression and the frequency of IFN-gamma producing cells; (3) a positive correlation amongst SLA specific, IFN-gamma or TNF-alpha and IL-10 producing lymphocytes with one another; and (4) a higher frequency of IL-10 producing, parasite specific (anti-SLA or anti-LACK), lymphocytes are correlated with a lower frequency of TNF-alpha producing monocytes, demonstrating an antigen specific delivery of IL-10 inducing negative regulation of monocyte activity.
Figures
Similar articles
-
Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis.Infect Immun. 2001 May;69(5):3232-9. doi: 10.1128/IAI.69.5.3232-3239.2001. Infect Immun. 2001. PMID: 11292745 Free PMC article.
-
T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure.Exp Parasitol. 1996 Nov;84(2):144-55. doi: 10.1006/expr.1996.0100. Exp Parasitol. 1996. PMID: 8932764
-
Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population.Scand J Immunol. 2006 Jan;63(1):70-8. doi: 10.1111/j.1365-3083.2005.01707.x. Scand J Immunol. 2006. PMID: 16398703
-
[Immunopathology of American tegumentary leishmaniasis].Acta Cient Venez. 1998;49(1):42-56. Acta Cient Venez. 1998. PMID: 10205916 Review. Spanish.
-
Immunopathology of leishmaniasis: an update.Int J Immunopathol Pharmacol. 2007 Jul-Sep;20(3):435-45. doi: 10.1177/039463200702000302. Int J Immunopathol Pharmacol. 2007. PMID: 17880757 Review.
Cited by
-
Ecto-nucleotidase activities of promastigotes from Leishmania (Viannia) braziliensis relates to parasite infectivity and disease clinical outcome.PLoS Negl Trop Dis. 2012;6(10):e1850. doi: 10.1371/journal.pntd.0001850. Epub 2012 Oct 11. PLoS Negl Trop Dis. 2012. PMID: 23071853 Free PMC article.
-
The immunotherapeutic role of regulatory T cells in Leishmania (Viannia) panamensis infection.J Immunol. 2014 Sep 15;193(6):2961-70. doi: 10.4049/jimmunol.1400728. Epub 2014 Aug 6. J Immunol. 2014. PMID: 25098291 Free PMC article.
-
Regulation of CD8+ T cell responses to infection with parasitic protozoa.Exp Parasitol. 2010 Nov;126(3):318-25. doi: 10.1016/j.exppara.2010.05.008. Epub 2010 May 21. Exp Parasitol. 2010. PMID: 20493842 Free PMC article. Review.
-
Conjunctival FOXP3 expression in trachoma: do regulatory T cells have a role in human ocular Chlamydia trachomatis infection?PLoS Med. 2006 Aug;3(8):e266. doi: 10.1371/journal.pmed.0030266. PLoS Med. 2006. PMID: 16881731 Free PMC article.
-
Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis.Parasite Immunol. 2007 May;29(5):251-8. doi: 10.1111/j.1365-3024.2007.00940.x. Parasite Immunol. 2007. PMID: 17430548 Free PMC article.
References
-
- Barral A, Pedral-Sampaio D, Grimaldi JG, et al. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg. 1991;44:536–46. - PubMed
-
- Mocci S, Coffman RL. Induction of a Th2 population from a polarized Leishmania-specific Th1 population by in vitro culture with IL-4. J Immunol. 1995;154:3779–87. - PubMed
-
- Sacks DL, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. - PubMed
-
- Liew FY, Xu D, Chan WL. Immune effector mechanism in parasitic infections. Immunol Lett. 1999;65:101–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

