Epidermal growth factor suppresses induction by progestin of the adhesion protein desmoplakin in T47D breast cancer cells
- PMID: 15084247
- PMCID: PMC400677
- DOI: 10.1186/bcr780
Epidermal growth factor suppresses induction by progestin of the adhesion protein desmoplakin in T47D breast cancer cells
Abstract
Introduction: Although the effects of progesterone on cell cycle progression are well known, its role in spreading and adhesion of breast cancer cells has not attracted much attention until recently. Indeed, by controlling cell adhesion proteins, progesterone may play a direct role in breast cancer invasion and metastasis. Progesterone has also been shown to modulate epidermal growth factor (EGF) effects in neoplasia, although EGF effects on progesterone pathways and targets are less well understood. In the present study we identify an effect of EGF on a progesterone target, namely desmoplakin.
Methods: Initially flow cytometry was used to establish the growing conditions and demonstrate that the T47D breast cancer cell line was responding to progesterone and EGF in a classical manner. Differential display RT-PCR was employed to identify differentially expressed genes affected by progesterone and EGF. Western and Northern blotting were used to verify interactions between EGF and progesterone in three breast cancer cell lines: T47D, MCF-7, and ZR-75.
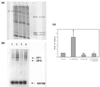
Results: We found the cell adhesion protein desmoplakin to be upregulated by progesterone - a process that was suppressed by EGF. This appears to be a general but not universal effect in breast cancer cell lines.
Conclusion: Our findings suggest that progesterone and EGF may play opposing roles in metastasis. They also suggest that desmoplakin may be a useful biomarker for mechanistic studies designed to analyze the crosstalk between EGF and progesterone dependent events. Our work may help to bridge the fields of metastasis and differentiation, and the mechanisms of steroid action.
Figures
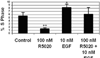
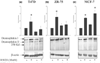
Similar articles
-
The angiogenic factor Cyr61 is induced by the progestin R5020 and is necessary for mammary adenocarcinoma cell growth.Endocrine. 2002 Jul;18(2):147-59. doi: 10.1385/ENDO:18:2:147. Endocrine. 2002. PMID: 12374462
-
Progesterone augments proliferation induced by epidermal growth factor in a feline mammary adenocarcinoma cell line.J Cell Biochem. 1991 Feb;45(2):196-206. doi: 10.1002/jcb.240450211. J Cell Biochem. 1991. PMID: 2055947
-
Regulation of fatty acid synthetase by progesterone in normal and tumoral human mammary glands.Rev Esp Fisiol. 1990 Mar;46(1):43-6. Rev Esp Fisiol. 1990. PMID: 2204092 Review.
-
Progesterone agonists and antagonists induce down- and up-regulation of estrogen receptors and estrogen inducible genes in human breast cancer cell lines.Int J Biol Markers. 1995 Jan-Mar;10(1):47-54. doi: 10.1177/172460089501000109. Int J Biol Markers. 1995. PMID: 7629427
-
Alternative splicing of the estrogen receptor primary transcript normally occurs in estrogen receptor positive tissues and cell lines.J Steroid Biochem Mol Biol. 1996 Jan;56(1-6 Spec No):99-105. doi: 10.1016/0960-0760(95)00227-8. J Steroid Biochem Mol Biol. 1996. PMID: 8603053 Review.
Cited by
-
Progesterone receptor A and c-Met mediates spheroids-endometrium attachment.Reprod Biol Endocrinol. 2009 Feb 16;7:14. doi: 10.1186/1477-7827-7-14. Reprod Biol Endocrinol. 2009. PMID: 19220894 Free PMC article.
-
Molecular genetics complexity impeding research progress in breast and ovarian cancers.Mol Clin Oncol. 2017 Jul;7(1):3-14. doi: 10.3892/mco.2017.1275. Epub 2017 May 29. Mol Clin Oncol. 2017. PMID: 28685067 Free PMC article.
-
Integrity of the LXXLL motif in Stat6 is required for the inhibition of breast cancer cell growth and enhancement of differentiation in the context of progesterone.BMC Cancer. 2014 Jan 8;14:10. doi: 10.1186/1471-2407-14-10. BMC Cancer. 2014. PMID: 24401087 Free PMC article.
-
Genomic-wide analysis of lymphatic metastasis-associated genes in human hepatocellular carcinoma.World J Gastroenterol. 2009 Jan 21;15(3):356-65. doi: 10.3748/wjg.15.356. World J Gastroenterol. 2009. PMID: 19140237 Free PMC article.
-
EGFR inhibition leads to enhanced desmosome assembly and cardiomyocyte cohesion via ROCK activation.JCI Insight. 2023 Mar 22;8(6):e163763. doi: 10.1172/jci.insight.163763. JCI Insight. 2023. PMID: 36795511 Free PMC article.
References
-
- Murphy LC, Dotzlaw H, Wong MSJ, Miller T, Mrockowski B, Gong Y, Murphy LJ. Epidermal growth factor: receptor and ligand expression in human breast cancer. Semin Cancer Biol. 1990;1:305–315. - PubMed
-
- Murphy LJ, Sutherland RL, Stead B, Murphy LC, Lazarus L. Progestin regulation of epidermal growth factor receptor in human mammary carcinoma cells. Cancer Res. 1986;46:728–734. - PubMed
-
- Arteaga CL, Osborne CK. Growth factors as mediators of estrogen/antioestrogen action in human breast cancer cells. In: Lippman ME and Dickson RB, editor. In Regulatory Mechanisms in Breast Cancer. Boston: Kluwer Academic Publishers; 1991. pp. 289–304.
-
- Dickson RB, Lippman ME. Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocr Rev. 1987;8:29–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

