Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox
- PMID: 15078924
- PMCID: PMC387704
- DOI: 10.1128/jvi.78.9.4433-4443.2004
Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox
Abstract
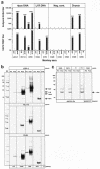
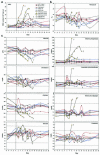
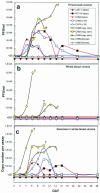
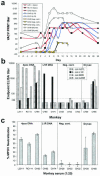
Two decades after a worldwide vaccination campaign was used to successfully eradicate naturally occurring smallpox, the threat of bioterrorism has led to renewed vaccination programs. In addition, sporadic outbreaks of human monkeypox in Africa and a recent outbreak of human monkeypox in the U.S. have made it clear that naturally occurring zoonotic orthopoxvirus diseases remain a public health concern. Much of the threat posed by orthopoxviruses could be eliminated by vaccination; however, because the smallpox vaccine is a live orthopoxvirus vaccine (vaccinia virus) administered to the skin, the vaccine itself can pose a serious health risk. Here, we demonstrate that rhesus macaques vaccinated with a DNA vaccine consisting of four vaccinia virus genes (L1R, A27L, A33R, and B5R) were protected from severe disease after an otherwise lethal challenge with monkeypox virus. Animals vaccinated with a single gene (L1R) which encodes a target of neutralizing antibodies developed severe disease but survived. This is the first demonstration that a subunit vaccine approach to smallpox-monkeypox immunization is feasible.
Figures

Similar articles
-
Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates.Virology. 2003 Feb 1;306(1):181-95. doi: 10.1016/s0042-6822(02)00038-7. Virology. 2003. PMID: 12620810 Free PMC article.
-
Systemically administered DNA and fowlpox recombinants expressing four vaccinia virus genes although immunogenic do not protect mice against the highly pathogenic IHD-J vaccinia strain.Virus Res. 2013 Dec 26;178(2):374-82. doi: 10.1016/j.virusres.2013.09.016. Epub 2013 Sep 16. Virus Res. 2013. PMID: 24050999 Free PMC article.
-
Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge.J Virol. 2008 Apr;82(7):3517-29. doi: 10.1128/JVI.01854-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199639 Free PMC article.
-
Recent advances in the study of live attenuated cell-cultured smallpox vaccine LC16m8.Vaccine. 2015 Nov 9;33(45):6106-11. doi: 10.1016/j.vaccine.2015.07.111. Epub 2015 Aug 28. Vaccine. 2015. PMID: 26319072 Free PMC article. Review.
-
Monkeypox virus and insights into its immunomodulatory proteins.Immunol Rev. 2008 Oct;225:96-113. doi: 10.1111/j.1600-065X.2008.00691.x. Immunol Rev. 2008. PMID: 18837778 Free PMC article. Review.
Cited by
-
Adsorption of recombinant poxvirus L1-protein to aluminum hydroxide/CpG vaccine adjuvants enhances immune responses and protection of mice from vaccinia virus challenge.Vaccine. 2013 Jan 2;31(2):319-26. doi: 10.1016/j.vaccine.2012.11.007. Epub 2012 Nov 12. Vaccine. 2013. PMID: 23153450 Free PMC article.
-
Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates.Vaccine. 2009 Dec 11;28(2):494-511. doi: 10.1016/j.vaccine.2009.09.133. Epub 2009 Oct 13. Vaccine. 2009. PMID: 19833247 Free PMC article.
-
Characterization and use of mammalian-expressed vaccinia virus extracellular membrane proteins for quantification of the humoral immune response to smallpox vaccines.Clin Vaccine Immunol. 2007 Aug;14(8):1032-44. doi: 10.1128/CVI.00050-07. Epub 2007 Jun 27. Clin Vaccine Immunol. 2007. PMID: 17596428 Free PMC article.
-
Global Research Trends on Monkeypox Virus: A Bibliometric and Visualized Study.Trop Med Infect Dis. 2022 Nov 28;7(12):402. doi: 10.3390/tropicalmed7120402. Trop Med Infect Dis. 2022. PMID: 36548657 Free PMC article. Review.
-
Mpox infection protects against re-challenge in rhesus macaques.Cell. 2023 Oct 12;186(21):4652-4661.e13. doi: 10.1016/j.cell.2023.08.023. Epub 2023 Sep 20. Cell. 2023. PMID: 37734373 Free PMC article.
References
-
- Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. - PMC - PubMed
-
- Centers for Disease Control and Prevention. 2003. Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. Morb. Mortal. Wkly. Rep. 52:537-540. - PubMed
-
- Centers for Disease Control and Prevention. 2003. Supplemental recommendations of adverse events following smallpox vaccine in the pre-event vaccination program: recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 52:282-284. - PubMed
-
- Centers for Disease Control and Prevention. 2003. Update: adverse events following civilian smallpox vaccination—United States, 2003. Morb. Mortal. Wkly. Rep. 52:419-420. - PubMed
-
- Centers for Disease Control and Prevention. 2003. Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Morb. Mortal. Wkly. Rep. 52:642-646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

