C-terminal repeat domain kinase I phosphorylates Ser2 and Ser5 of RNA polymerase II C-terminal domain repeats
- PMID: 15047695
- PMCID: PMC2680323
- DOI: 10.1074/jbc.M402218200
C-terminal repeat domain kinase I phosphorylates Ser2 and Ser5 of RNA polymerase II C-terminal domain repeats
Abstract
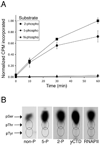
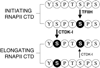
The C-terminal repeat domain (CTD) of the largest subunit of RNA polymerase II is composed of tandem heptad repeats with consensus sequence Tyr1-Ser2-Pro3-Thr4-Ser5-Pro6-Ser7. In yeast, this heptad sequence is repeated about 26 times, and it becomes hyperphosphorylated during transcription predominantly at serines 2 and 5. A network of kinases and phosphatases combine to determine the CTD phosphorylation pattern. We sought to determine the positional specificity of phosphorylation by yeast CTD kinase-I (CTDK-I), an enzyme implicated in various nuclear processes including elongation and pre-mRNA 3'-end formation. Toward this end, we characterized monoclonal antibodies commonly employed to study CTD phosphorylation patterns and found that the H5 monoclonal antibody reacts with CTD species phosphorylated at Ser2 and/or Ser5. We therefore used antibody-independent methods to study CTDK-I, and we found that CTDK-I phosphorylates Ser5 of the CTD if the CTD substrate is either unphosphorylated or prephosphorylated at Ser2. When Ser5 is already phosphorylated, CTDK-I phosphorylates Ser2 of the CTD. We also observed that CTDK-I efficiently generates doubly phosphorylated CTD repeats; CTD substrates that already contain Ser2-PO(4) or Ser5-PO(4) are more readily phosphorylated CTDK-I than unphosphorylby ated CTD substrates.
Figures




Similar articles
-
Characterization of the residues phosphorylated in vitro by different C-terminal domain kinases.J Biol Chem. 1998 Mar 20;273(12):6769-75. doi: 10.1074/jbc.273.12.6769. J Biol Chem. 1998. PMID: 9506978
-
Phosphorylation causes a conformational change in the carboxyl-terminal domain of the mouse RNA polymerase II largest subunit.J Biol Chem. 1991 Feb 5;266(4):2297-302. J Biol Chem. 1991. PMID: 1989983
-
Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription.Biochim Biophys Acta. 2013 Jan;1829(1):55-62. doi: 10.1016/j.bbagrm.2012.08.013. Epub 2012 Sep 7. Biochim Biophys Acta. 2013. PMID: 22982363 Review.
-
Identification of phosphorylation sites in the repetitive carboxyl-terminal domain of the mouse RNA polymerase II largest subunit.J Biol Chem. 1991 Feb 5;266(4):2290-6. J Biol Chem. 1991. PMID: 1899239
-
Tyrosine-1 and threonine-4 phosphorylation marks complete the RNA polymerase II CTD phospho-code.RNA Biol. 2012 Sep;9(9):1144-6. doi: 10.4161/rna.21726. Epub 2012 Sep 1. RNA Biol. 2012. PMID: 22960391 Free PMC article. Review.
Cited by
-
ChIP-seq and RNA-seq methods to study circadian control of transcription in mammals.Methods Enzymol. 2015;551:285-321. doi: 10.1016/bs.mie.2014.10.059. Epub 2014 Dec 26. Methods Enzymol. 2015. PMID: 25662462 Free PMC article.
-
Emerging Views on the CTD Code.Genet Res Int. 2012;2012:347214. doi: 10.1155/2012/347214. Epub 2012 Feb 26. Genet Res Int. 2012. PMID: 22567385 Free PMC article.
-
Expanding the functional repertoire of CTD kinase I and RNA polymerase II: novel phosphoCTD-associating proteins in the yeast proteome.Biochemistry. 2004 Dec 21;43(50):15702-19. doi: 10.1021/bi048364h. Biochemistry. 2004. PMID: 15595826 Free PMC article.
-
Progression through the RNA polymerase II CTD cycle.Mol Cell. 2009 Nov 25;36(4):541-6. doi: 10.1016/j.molcel.2009.10.019. Mol Cell. 2009. PMID: 19941815 Free PMC article. Review.
-
Selective Kinase Inhibition Shows That Bur1 (Cdk9) Phosphorylates the Rpb1 Linker In Vivo.Mol Cell Biol. 2019 Jul 16;39(15):e00602-18. doi: 10.1128/MCB.00602-18. Print 2019 Aug 1. Mol Cell Biol. 2019. PMID: 31085683 Free PMC article.
References
-
- Cadena DL, Dahmus ME. J. Biol. Chem. 1987;262:12468–12474. - PubMed
-
- Laybourn PJ, Dahmus ME. J. Biol. Chem. 1989;264:6693–6698. - PubMed
-
- Payne JM, Laybourn PJ, Dahmus ME. J. Biol. Chem. 1989;264:19621–19629. - PubMed
-
- Weeks JR, Hardin SE, Shen J, Lee JM, Greenleaf AL. Genes Dev. 1993;7:2329–2344. - PubMed
-
- Kang ME, Dahmus ME. J. Biol. Chem. 1993;268:25033–25040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

