Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail
- PMID: 15016865
- PMCID: PMC371074
- DOI: 10.1128/jvi.78.7.3429-3435.2004
Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail
Abstract
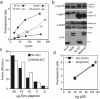
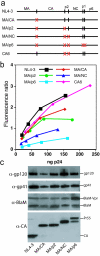
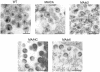
Retrovirus particles are not infectious until they undergo proteolytic maturation to form a functional core. Here we report a link between human immunodeficiency virus type 1 (HIV-1) core maturation and the ability of the virus to fuse with target cells. Using a recently developed reporter assay of HIV-1 virus-cell fusion, we show that immature HIV-1 particles are 5- to 10-fold less active for fusion with target cells than are mature virions. The fusion of mature and immature virions was rendered equivalent by truncating the gp41 cytoplasmic domain or by pseudotyping viruses with the glycoprotein of vesicular stomatitis virus. An analysis of a panel of mutants containing mutated cleavage sites indicated that HIV-1 fusion competence is activated by the cleavage of Gag at any site between the MA and NC segments and not as an indirect consequence of an altered core structure. These results suggest a mechanism by which binding of the gp41 cytoplasmic tail to Gag within immature HIV-1 particles inhibits Env conformational changes on the surface of the virion that are required for membrane fusion. This "inside-out" regulation of HIV-1 fusion could play an important role in the virus life cycle by preventing the entry of immature, noninfectious particles.
Figures
Similar articles
-
Maturation-dependent human immunodeficiency virus type 1 particle fusion requires a carboxyl-terminal region of the gp41 cytoplasmic tail.J Virol. 2007 Sep;81(18):9999-10008. doi: 10.1128/JVI.00592-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609279 Free PMC article.
-
Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions.J Virol. 1996 Jan;70(1):341-51. doi: 10.1128/JVI.70.1.341-351.1996. J Virol. 1996. PMID: 8523546 Free PMC article.
-
A mutation in the human immunodeficiency virus type 1 Gag protein destabilizes the interaction of the envelope protein subunits gp120 and gp41.J Virol. 2006 Mar;80(5):2405-17. doi: 10.1128/JVI.80.5.2405-2417.2006. J Virol. 2006. PMID: 16474147 Free PMC article.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
-
The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation.Viruses. 2020 May 16;12(5):548. doi: 10.3390/v12050548. Viruses. 2020. PMID: 32429351 Free PMC article. Review.
Cited by
-
The Assembly of HTLV-1-How Does It Differ from HIV-1?Viruses. 2024 Sep 27;16(10):1528. doi: 10.3390/v16101528. Viruses. 2024. PMID: 39459862 Free PMC article. Review.
-
Detailed topology mapping reveals substantial exposure of the "cytoplasmic" C-terminal tail (CTT) sequences in HIV-1 Env proteins at the cell surface.PLoS One. 2013 May 27;8(5):e65220. doi: 10.1371/journal.pone.0065220. Print 2013. PLoS One. 2013. PMID: 23724133 Free PMC article.
-
HIV-1 Gag processing intermediates trans-dominantly interfere with HIV-1 infectivity.J Biol Chem. 2009 Oct 23;284(43):29692-703. doi: 10.1074/jbc.M109.027144. Epub 2009 Aug 7. J Biol Chem. 2009. PMID: 19666477 Free PMC article.
-
Role of the C-terminal domain of the HIV-1 glycoprotein in cell-to-cell viral transmission between T lymphocytes.Retrovirology. 2010 May 12;7:43. doi: 10.1186/1742-4690-7-43. Retrovirology. 2010. PMID: 20459872 Free PMC article.
-
Foreign glycoproteins can be actively recruited to virus assembly sites during pseudotyping.J Virol. 2009 May;83(9):4060-7. doi: 10.1128/JVI.02425-08. Epub 2009 Feb 18. J Virol. 2009. PMID: 19224995 Free PMC article.
References
-
- Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

