Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria
- PMID: 14998421
- PMCID: PMC1884452
- DOI: 10.1046/j.1365-2125.2003.02004.x
Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria
Abstract
Aims: To study the population pharmacokinetics of piperaquine after co-administration with dihydroartemisinin in uncomplicated malaria.
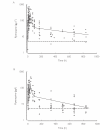
Methods: The disposition of piperaquine was studied in 85 Cambodian patients with uncomplicated falciparum or vivax malaria treated with the piperaquine-dihydroartemisinin coformulation Artekin. All patients were given Artekin orally at 0, 6, 24 and 32 h with a total piperaquine dose of 32-35 mg base kg-1. Adults were given tablets while children received either tablets or a dispersible granule formulation. Patients underwent either intensive (17-19 samples) or sparse (2-5 samples) blood sampling schedules over 35 days and clinical/parasitological follow-up over > 28 days. Piperaquine in plasma was quantified by high performance liquid chromatography.
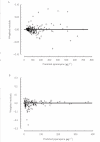
Results: All patients achieved fever clearance within 24 h and parasite clearance within 72 h. The 28-day cure rate was 97% in adults and 98% in children. A covariate-free two-compartment population model with first-order absorption and elimination gave the most robust representation of the plasma concentration-time data in both adults and children. In adults (n = 38), the median (interquartile range) derived pharmacokinetic descriptors CL/F, Vss/F and t1/2,z were 0.9 l h-1 kg-1 (0.79-1.02 l h-1 kg-1), 574 l kg-1(371-711 l kg-1) and 23 days (19-28 days), respectively. In children (n = 47), corresponding values were 1.8 l h-1 kg-1 (1.29-2.3 l h-1 kg-1), 614 l kg-1 (332-1205 l kg-1) and 14 days (10-18 days), respectively.
Conclusions: Piperaquine is a highly lipid-soluble drug with a large Vss/F, long t1/2,z and a clearance that is markedly higher in children than in adults.
Figures

Similar articles
-
Pharmacokinetics and efficacy of piperaquine and chloroquine in Melanesian children with uncomplicated malaria.Antimicrob Agents Chemother. 2008 Jan;52(1):237-43. doi: 10.1128/AAC.00555-07. Epub 2007 Oct 29. Antimicrob Agents Chemother. 2008. PMID: 17967917 Free PMC article. Clinical Trial.
-
Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis.PLoS Med. 2017 Jan 10;14(1):e1002212. doi: 10.1371/journal.pmed.1002212. eCollection 2017 Jan. PLoS Med. 2017. PMID: 28072872 Free PMC article.
-
Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria.Br J Clin Pharmacol. 2004 Jan;57(1):93-9. doi: 10.1046/j.1365-2125.2003.01962.x. Br J Clin Pharmacol. 2004. PMID: 14678346 Free PMC article. Clinical Trial.
-
Dihydroartemisinin/Piperaquine: a review of its use in the treatment of uncomplicated Plasmodium falciparum malaria.Drugs. 2012 May 7;72(7):937-61. doi: 10.2165/11203910-000000000-00000. Drugs. 2012. PMID: 22515619 Review.
-
Artemisinin-based combination therapy for treating uncomplicated malaria.Cochrane Database Syst Rev. 2009 Jul 8;2009(3):CD007483. doi: 10.1002/14651858.CD007483.pub2. Cochrane Database Syst Rev. 2009. PMID: 19588433 Free PMC article. Review.
Cited by
-
Efficacy of Artemether-Lumefantrine and Dihydroartemisinin-Piperaquine for the Treatment of Uncomplicated Plasmodium falciparum Malaria among Children in Western Kenya, 2016 to 2017.Antimicrob Agents Chemother. 2022 Sep 20;66(9):e0020722. doi: 10.1128/aac.00207-22. Epub 2022 Aug 29. Antimicrob Agents Chemother. 2022. PMID: 36036611 Free PMC article.
-
Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy.PLoS One. 2014 Jan 17;9(1):e84976. doi: 10.1371/journal.pone.0084976. eCollection 2014. PLoS One. 2014. PMID: 24465458 Free PMC article.
-
-Pharmacokinetics of antimalarial drugs used to treat uncomplicated malaria in breastfeeding mother-infant pairs: An observational pharmacokinetic study.Wellcome Open Res. 2023 Aug 10;8:12. doi: 10.12688/wellcomeopenres.18512.1. eCollection 2023. Wellcome Open Res. 2023. PMID: 37744730 Free PMC article.
-
Effect of coadministered fat on the tolerability, safety, and pharmacokinetic properties of dihydroartemisinin-piperaquine in Papua New Guinean children with uncomplicated malaria.Antimicrob Agents Chemother. 2014 Oct;58(10):5784-94. doi: 10.1128/AAC.03314-14. Epub 2014 Jul 21. Antimicrob Agents Chemother. 2014. PMID: 25049242 Free PMC article. Clinical Trial.
-
Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda.PLoS One. 2008 Jun 11;3(6):e2390. doi: 10.1371/journal.pone.0002390. PLoS One. 2008. PMID: 18545692 Free PMC article. Clinical Trial.
References
-
- Chen L, Qu FY, Zhou YC. Field observations on the antimalarial piperaquine. Chin Med J (Engl) 1982;95:281–6. - PubMed
-
- Chen L. Recent studies on antimalarial efficacy of piperaquine and hydroxypiperaquine. Chin Med J (Engl) 1991;104:161–3. - PubMed
-
- Fan B, Zhao W, Ma X, et al. In vitro sensitivity of Plasmodium falciparum to chloroquine, piperaquine, pyronaridine and artesunate in Yuxi prefecture of Yunnan province. Chin J Parasitol Parasit Dis. 1998;16:460–2. - PubMed
-
- Lan CX, Lin X, Huang ZS, Chen YS, Guo RN. In vivo sensitivity of Plasmodium falciparum to piperaquine phosphate assayed in Linshui and Baisha counties, Hainan Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1989;7:163–5. - PubMed
-
- Yang H, Liu D, Huang K, et al. Assay of sensitivity of Plasmodium falciparum to chloroquine, amodiaquine, piperaquine, mefloquine and quinine in Yunnan province. Chin J Parasitol Parasit Dis. 1999;17:43–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

