Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission
- PMID: 14985430
- PMCID: PMC6730409
- DOI: 10.1523/JNEUROSCI.4084-03.2004
Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission
Abstract
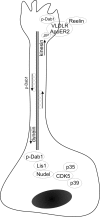
Neuronal migration and positioning in the developing brain require the coordinated interaction of multiple cellular signaling pathways. The extracellular signaling molecule Reelin and the cytoplasmic serine/threonine kinase Cdk5 (cyclin-dependent kinase 5) are both required for normal neuronal positioning, lamination of the neocortex, and foliation of the cerebellum. They also modulate synaptic transmission in the adult brain. It is not known, however, to what extent Cdk5 participates in Reelin signaling and whether both pathways interact on the genetic or biochemical level. We have used genetically altered mice to generate compound functional defects of Reelin and Cdk5 signaling. Differential neurohistochemical staging combined with the biochemical analysis of Reelin- and Cdk5-dependent signaling in primary embryonic neurons and electrophysiology in hippocampal slices reveals evidence for genetic and functional interaction between both pathways. Inhibition of Reelin or Cdk5 signaling had no discernible biochemical effect on each other. Taken together, these findings suggest that both pathways function together in a parallel, rather than a simple, linear manner to coordinate neuronal migration and neurotransmission in the developing and mature brain.
Figures


Similar articles
-
Cyclin-dependent kinase 5 phosphorylates disabled 1 independently of Reelin signaling.J Neurosci. 2002 Jun 15;22(12):4869-77. doi: 10.1523/JNEUROSCI.22-12-04869.2002. J Neurosci. 2002. PMID: 12077184 Free PMC article.
-
Reelin signaling and Cdk5 in the control of neuronal positioning.Mol Neurobiol. 2002 Oct-Dec;26(2-3):153-66. doi: 10.1385/MN:26:2-3:153. Mol Neurobiol. 2002. PMID: 12428753 Review.
-
Modulation of Reelin signaling by Cyclin-dependent kinase 5.Brain Res. 2007 Apr 6;1140:84-95. doi: 10.1016/j.brainres.2006.01.121. Epub 2006 Mar 10. Brain Res. 2007. PMID: 16529723
-
Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain.Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2764-9. doi: 10.1073/pnas.051628498. Epub 2001 Feb 13. Proc Natl Acad Sci U S A. 2001. PMID: 11226314 Free PMC article.
-
Reelin and apoE actions on signal transduction, synaptic function and memory formation.Neuron Glia Biol. 2008 Aug;4(3):259-70. doi: 10.1017/S1740925X09990184. Epub 2009 Aug 13. Neuron Glia Biol. 2008. PMID: 19674510 Review.
Cited by
-
Serine phosphorylation regulates disabled-1 early isoform turnover independently of Reelin.Cell Signal. 2011 Mar;23(3):555-65. doi: 10.1016/j.cellsig.2010.11.007. Epub 2010 Nov 25. Cell Signal. 2011. PMID: 21111810 Free PMC article.
-
The Role of Reelin Signaling in Alzheimer's Disease.Mol Neurobiol. 2016 Oct;53(8):5692-700. doi: 10.1007/s12035-015-9459-9. Epub 2015 Oct 21. Mol Neurobiol. 2016. PMID: 26491027 Review.
-
Cyclin-dependent kinase 5 activates guanine nucleotide exchange factor GIV/Girdin to orchestrate migration-proliferation dichotomy.Proc Natl Acad Sci U S A. 2015 Sep 1;112(35):E4874-83. doi: 10.1073/pnas.1514157112. Epub 2015 Aug 18. Proc Natl Acad Sci U S A. 2015. PMID: 26286990 Free PMC article.
-
Genome-wide target analysis of NEUROD2 provides new insights into regulation of cortical projection neuron migration and differentiation.BMC Genomics. 2015 Sep 5;16:681. doi: 10.1186/s12864-015-1882-9. BMC Genomics. 2015. PMID: 26341353 Free PMC article.
-
From clusters to stripes: the developmental origins of adult cerebellar compartmentation.Cerebellum. 2006;5(2):77-88. doi: 10.1080/14734220600804668. Cerebellum. 2006. PMID: 16818382 Review.
References
-
- Arnaud L, Ballif BA, Forster E, Cooper JA (2003) Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr Biol 13: 9-17. - PubMed
-
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D'Arcangelo G, Clark GD (2003) Interaction of reelin signaling and Lis1 in brain development. Nat Genet 35: 270-276. - PubMed
-
- Banker G, Goslin K (1988) Developments in neuronal cell culture. Nature 336: 185-186. - PubMed
-
- Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E (1993) Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett 336: 417-424. - PubMed
-
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J (2002) Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem 277: 49958-49964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
