Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway
- PMID: 14982863
- PMCID: PMC1614723
- DOI: 10.1016/S0002-9440(10)63197-5
Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway
Abstract
Ectodermal organs are composed of keratinocytes organized in different ways during induction, morphogenesis, differentiation, and regenerative stages. We hypothesize that an imbalance of fundamental signaling pathways should affect multiple ectodermal organs in a spatio-temporal-dependent manner. We produced a K14-Noggin transgenic mouse to modulate bone morphogenic protein (BMP) activity and test the extent of this hypothesis. We observed thickened skin epidermis, increased hair density, altered hair types, faster anagen re-entry, and formation of compound vibrissa follicles. The eyelid opening was smaller and ectopic cilia formed at the expense of Meibomian glands. In the distal limb, there were agenesis and hyperpigmentation of claws, interdigital webbing, reduced footpads, and trans-differentiation of sweat glands into hairs. The size of external genitalia increased in both sexes, but they remained fertile. We conclude that modulation of BMP activity can affect the number of ectodermal organs by acting during induction stages, influence the size and shape by acting during morphogenesis stages, change phenotypes by acting during differentiation stages, and facilitate new growth by acting during regeneration stages. Therefore during organogenesis, BMP antagonists can produce a spectrum of phenotypes in a stage-dependent manner by adjusting the level of BMP activity. The distinction between phenotypic variations and pathological changes is discussed.
Figures
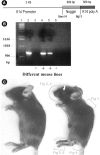
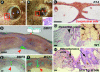
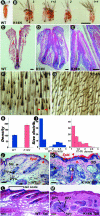


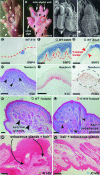

Similar articles
-
Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling.Am J Pathol. 2004 Sep;165(3):729-40. doi: 10.1016/S0002-9440(10)63336-6. Am J Pathol. 2004. PMID: 15331398 Free PMC article.
-
Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression.Development. 2007 Jan;134(1):117-25. doi: 10.1242/dev.02708. Development. 2007. PMID: 17164417
-
Noggin overexpression inhibits eyelid opening by altering epidermal apoptosis and differentiation.EMBO J. 2003 Jun 16;22(12):2992-3003. doi: 10.1093/emboj/cdg291. EMBO J. 2003. PMID: 12805214 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Evo-Devo of amniote integuments and appendages.Int J Dev Biol. 2004;48(2-3):249-70. doi: 10.1387/ijdb.041825pw. Int J Dev Biol. 2004. PMID: 15272390 Free PMC article. Review.
Cited by
-
Differential modularity of the mammalian Engrailed 1 enhancer network directs sweat gland development.PLoS Genet. 2023 Feb 6;19(2):e1010614. doi: 10.1371/journal.pgen.1010614. eCollection 2023 Feb. PLoS Genet. 2023. PMID: 36745673 Free PMC article.
-
Defining Key Genes Regulating Morphogenesis of Apocrine Sweat Gland in Sheepskin.Front Genet. 2019 Jan 30;9:739. doi: 10.3389/fgene.2018.00739. eCollection 2018. Front Genet. 2019. PMID: 30761184 Free PMC article.
-
The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland.Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1938-78. doi: 10.1167/iovs.10-6997c. Print 2011 Mar. Invest Ophthalmol Vis Sci. 2011. PMID: 21450915 Free PMC article. Review. No abstract available.
-
A genetic basis of variation in eccrine sweat gland and hair follicle density.Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):9932-7. doi: 10.1073/pnas.1511680112. Epub 2015 Jul 20. Proc Natl Acad Sci U S A. 2015. PMID: 26195765 Free PMC article.
-
ID1 and CEBPA coordinate epidermal progenitor cell differentiation.Development. 2022 Nov 15;149(22):dev201262. doi: 10.1242/dev.201262. Epub 2022 Nov 16. Development. 2022. PMID: 36330928 Free PMC article.
References
-
- Chuong C-M, editor. Austin: Landes Bioscience,; Molecular Basis of Epithelial Appendage Morphogenesis. 1998
-
- Wisniewski SA, Kobielak A, Trzeciak WH, Kobielak K. Recent advances in understanding of the molecular basis of anhidrotic ectodermal dysplasia: discovery of a ligand, ectodysplasin A and its two receptors. J Appl Genet. 2002;43:97–107. - PubMed
-
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in HF induction. Nat Genet. 1999;22:370–374. - PubMed
-
- Brunner HG, Hamel BC, Bokhoven HVH. P63 gene mutations and human developmental syndromes. Am J Med Genet. 2002;112:284–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR042177-07S1/AR/NIAMS NIH HHS/United States
- 1 P03 DK48522/DK/NIDDK NIH HHS/United States
- AR47364/AR/NIAMS NIH HHS/United States
- R01 AR047364-01A2/AR/NIAMS NIH HHS/United States
- R01 AR042177/AR/NIAMS NIH HHS/United States
- R01 AR047364/AR/NIAMS NIH HHS/United States
- R01 AR042177-08S1/AR/NIAMS NIH HHS/United States
- R01 AR042177-09/AR/NIAMS NIH HHS/United States
- P30 DK048522/DK/NIDDK NIH HHS/United States
- R01 AR047364-02/AR/NIAMS NIH HHS/United States
- R01 AR042177-08/AR/NIAMS NIH HHS/United States
- AR42177/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

