Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal
- PMID: 14981093
- PMCID: PMC2172171
- DOI: 10.1083/jcb.200310138
Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal
Abstract
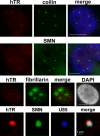
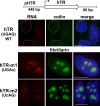
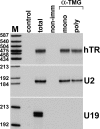
Telomerase is a ribonucleoprotein reverse transcriptase that uses its RNA component as a template for synthesis of telomeric DNA repeats at the ends of linear eukaryotic chromosomes. Here, fluorescence in situ hybridization demonstrates that in HeLa cancer cells, human telomerase RNA (hTR) accumulates in the nucleoplasmic Cajal bodies (CBs). Localization of transiently expressed hTR to CBs is supported by a short sequence motif (411-UGAG-414) that is located in the 3'-terminal box H/ACA RNA-like domain of hTR and that is structurally and functionally indistinguishable from the CB-specific localization signal of box H/ACA small CB-specific RNAs. In synchronized HeLa cells, hTR shows the most efficient accumulation in CBs during S phase, when telomeres are most likely synthesized. CBs may function in post-transcriptional maturation (e.g., cap hypermethylation of hTR), but they may also play a role in the assembly and/or function of telomerase holoenzyme.
Figures

Comment in
-
Can telomerase be put in its place?J Cell Biol. 2004 Mar 1;164(5):637-9. doi: 10.1083/jcb.200401152. J Cell Biol. 2004. PMID: 14993231 Free PMC article.
Similar articles
-
Structural and functional characterization of human telomerase RNA processing and cajal body localization signals.Mol Cell. 2007 Sep 21;27(6):869-81. doi: 10.1016/j.molcel.2007.07.017. Mol Cell. 2007. PMID: 17889661
-
A common sequence motif determines the Cajal body-specific localization of box H/ACA scaRNAs.EMBO J. 2003 Aug 15;22(16):4283-93. doi: 10.1093/emboj/cdg394. EMBO J. 2003. PMID: 12912925 Free PMC article.
-
The snoRNA domain of vertebrate telomerase RNA functions to localize the RNA within the nucleus.RNA. 2001 Dec;7(12):1833-44. RNA. 2001. PMID: 11780638 Free PMC article.
-
RNA structure and function in C/D and H/ACA s(no)RNPs.Curr Opin Struct Biol. 2004 Jun;14(3):335-43. doi: 10.1016/j.sbi.2004.05.006. Curr Opin Struct Biol. 2004. PMID: 15193314 Review.
-
Biogenesis and intranuclear trafficking of human box C/D and H/ACA RNPs.Cold Spring Harb Symp Quant Biol. 2006;71:407-17. doi: 10.1101/sqb.2006.71.025. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381323 Review.
Cited by
-
Regulation of human telomerase in homeostasis and disease.Nat Rev Mol Cell Biol. 2020 Jul;21(7):384-397. doi: 10.1038/s41580-020-0234-z. Epub 2020 Apr 2. Nat Rev Mol Cell Biol. 2020. PMID: 32242127 Free PMC article. Review.
-
Pof8 is a La-related protein and a constitutive component of telomerase in fission yeast.Nat Commun. 2018 Feb 8;9(1):587. doi: 10.1038/s41467-017-02284-8. Nat Commun. 2018. PMID: 29422664 Free PMC article.
-
Telomerase Regulation from Beginning to the End.Genes (Basel). 2016 Sep 14;7(9):64. doi: 10.3390/genes7090064. Genes (Basel). 2016. PMID: 27649246 Free PMC article. Review.
-
Telomerase RNA processing: Implications for human health and disease.Stem Cells. 2020 Sep 1:10.1002/stem.3270. doi: 10.1002/stem.3270. Online ahead of print. Stem Cells. 2020. PMID: 32875693 Free PMC article. Review.
-
The structure and function of small nucleolar ribonucleoproteins.Nucleic Acids Res. 2007;35(5):1452-64. doi: 10.1093/nar/gkl1172. Epub 2007 Feb 6. Nucleic Acids Res. 2007. PMID: 17284456 Free PMC article. Review.
References
-
- Chen, J.L., M.A. Blasco, and C.W. Greider. 2000. Secondary structure of vertebrate telomerase RNA. Cell. 100:503–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

