Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes
- PMID: 14963170
- PMCID: PMC369236
- DOI: 10.1128/jvi.78.5.2627-2631.2004
Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes
Abstract
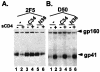
We investigated how the broadly neutralizing monoclonal antibody 2F5 affects the human immunodeficiency virus type 1 envelope glycoprotein as it undergoes receptor-induced conformational changes and show that 2F5 binds both native and fusion-intermediate conformations, suggesting inhibition of a late step in virus entry. We also demonstrate conformational changes in the C heptad of gp41.
Figures

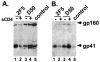
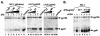
Similar articles
-
Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion.J Virol. 2002 Dec;76(23):12123-34. doi: 10.1128/jvi.76.23.12123-12134.2002. J Virol. 2002. PMID: 12414953 Free PMC article.
-
Multifaceted consequences of anti-gp41 monoclonal antibody 2F5 binding to HIV type 1 virions.AIDS Res Hum Retroviruses. 1995 Jun;11(6):687-96. doi: 10.1089/aid.1995.11.687. AIDS Res Hum Retroviruses. 1995. PMID: 7576928
-
A conformation-specific monoclonal antibody reacting with fusion-active gp41 from the human immunodeficiency virus type 1 envelope glycoprotein.J Virol. 1998 Dec;72(12):10213-7. doi: 10.1128/JVI.72.12.10213-10217.1998. J Virol. 1998. PMID: 9811763 Free PMC article.
-
Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
-
Structural and functional features of the HIV envelope glycoprotein and considerations for vaccine development.Biotechnology. 1990;14:81-110. doi: 10.1016/b978-0-409-90116-0.50013-3. Biotechnology. 1990. PMID: 1691670 Review. No abstract available.
Cited by
-
Global Increases in Human Immunodeficiency Virus Neutralization Sensitivity Due to Alterations in the Membrane-Proximal External Region of the Envelope Glycoprotein Can Be Minimized by Distant State 1-Stabilizing Changes.J Virol. 2022 Apr 13;96(7):e0187821. doi: 10.1128/jvi.01878-21. Epub 2022 Mar 15. J Virol. 2022. PMID: 35289647 Free PMC article.
-
Time frames for neutralization during the human immunodeficiency virus type 1 entry phase, as monitored in synchronously infected cell cultures.J Virol. 2007 Apr;81(7):3525-34. doi: 10.1128/JVI.02293-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251303 Free PMC article.
-
Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41.PLoS Pathog. 2010 Nov 18;6(11):e1001195. doi: 10.1371/journal.ppat.1001195. PLoS Pathog. 2010. PMID: 21124990 Free PMC article.
-
Effect of epitope position on neutralization by anti-human immunodeficiency virus monoclonal antibody 2F5.J Virol. 2006 Mar;80(5):2539-47. doi: 10.1128/JVI.80.5.2539-2547.2006. J Virol. 2006. PMID: 16474160 Free PMC article.
-
Immunogenicity of recombinant human immunodeficiency virus type 1-like particles expressing gp41 derivatives in a pre-fusion state.Vaccine. 2007 Jun 28;25(27):5102-14. doi: 10.1016/j.vaccine.2006.09.071. Epub 2006 Oct 9. Vaccine. 2007. PMID: 17055621 Free PMC article.
References
-
- Barbato, G., E. Bianchi, P. Ingallinella, W. H. Hurni, M. D. Miller, G. Ciliberto, R. Cortese, R. Bazzo, J. W. Shiver, and A. Pessi. 2003. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J. Mol. Biol. 330:1101-1115. - PubMed
-
- Biron, Z., S. Khare, A. O. Samson, Y. Hayek, F. Naider, and J. Anglister. 2002. A monomeric 3(10)-helix is formed in water by a 13-residue peptide representing the neutralizing determinant of HIV-1 on gp41. Biochemistry 41:12687-12696. - PubMed
-
- Carr, C. M., and P. S. Kim. 1993. A spring-loaded mechanism for the conformational changes of influenza hemagglutinin. Cell 73:823-832. - PubMed
-
- Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. - PubMed
-
- Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

