Cell cycle requirements for transduction by foamy virus vectors compared to those of oncovirus and lentivirus vectors
- PMID: 14963129
- PMCID: PMC369213
- DOI: 10.1128/jvi.78.5.2327-2335.2004
Cell cycle requirements for transduction by foamy virus vectors compared to those of oncovirus and lentivirus vectors
Abstract
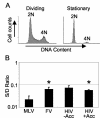
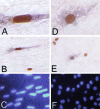
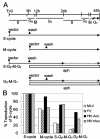
Retroviral vectors based on foamy viruses (FV) are efficient gene delivery vehicles for therapeutic and research applications. While previous studies have shown that FV vectors transduce quiescent cell cultures more efficiently than oncoviral vectors, their specific cell cycle requirements have not been determined. Here we compare the transduction frequencies of FV vectors with those of onco- and lentiviral vectors in nondividing and dividing normal human fibroblasts by several methods. FV vectors transduced serum-deprived fibroblast cultures more efficiently than oncoretroviral vectors and at rates comparable to those of lentiviral vectors. However, in these cultures FV vectors only transduced a subpopulation of proliferating cells, as determined by bromodeoxyuridine staining for DNA synthesis. In contrast to lentiviral vectors, FV vectors were unable to transduce human fibroblasts arrested by aphidicolin (G(1)/S phase) or gamma-irradiation (G(2) phase), and a partial cell cycle that included mitosis but not DNA synthesis was required. We could not determine if mitosis facilitated nuclear entry of FV vectors, since cell-free vector preparations contained long terminal repeat circles, precluding their use as nuclear markers. In contrast to oncoviral vectors, both FV and lentiviral vectors efficiently transduced G(0) fibroblasts that were later stimulated to divide. In the case of FV vectors, this was due to the persistence of a stable transduction intermediate in quiescent cells. Our findings support the use of FV vectors as a safe and effective alternative to lentiviral vectors for ex vivo transduction of stem cells that are quiescent during culture but divide following transplantation.
Figures



References
-
- Alexander, I. E., D. W. Russell, and A. D. Miller. 1997. Transfer of contaminants in adeno-associated virus vector stocks can mimic transduction and lead to artifactual results. Hum. Gene Ther. 8:1911-1920. - PubMed
-
- Bieniasz, P. D., O. Erlwein, A. Aguzzi, A. Rethwilm, and M. O. McClure. 1997. Gene transfer using replication-defective human foamy virus vectors. Virology 235:65-72. - PubMed
-
- Brown, P., G. Nemo, and D. C. Gajdusek. 1978. Human foamy virus: further characterization, seroepidemiology, and relationship to chimpanzee foamy viruses. J. Infect. Dis. 137:421-427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

