Pre-steady-state kinetics shows differences in processing of various DNA lesions by Escherichia coli formamidopyrimidine-DNA glycosylase
- PMID: 14769949
- PMCID: PMC373384
- DOI: 10.1093/nar/gkh237
Pre-steady-state kinetics shows differences in processing of various DNA lesions by Escherichia coli formamidopyrimidine-DNA glycosylase
Abstract
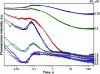
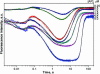
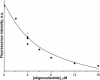
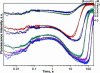
Formamidopyrimidine-DNA-glycosylase (Fpg protein, MutM) catalyses excision of 8-oxoguanine (8-oxoG) and other oxidatively damaged purines from DNA in a glycosylase/apurinic/apyrimidinic-lyase reaction. We report pre-steady-state kinetic analysis of Fpg action on oligonucleotide duplexes containing 8-oxo-2'-deoxyguanosine, natural abasic site or tetrahydrofuran (an uncleavable abasic site analogue). Monitoring Fpg intrinsic tryptophan fluorescence in stopped-flow experiments reveals multiple conformational transitions in the protein molecule during the catalytic cycle. At least four and five conformational transitions occur in Fpg during the interaction with abasic and 8-oxoG-containing substrates, respectively, within 2 ms to 10 s time range. These transitions reflect the stages of enzyme binding to DNA and lesion recognition with the mutual adjustment of DNA and enzyme structures to achieve catalytically competent conformation. Unlike these well-defined binding steps, catalytic stages are not associated with discernible fluorescence events. Only a single conformational change is detected for the cleavable substrates at times exceeding 10 s. The data obtained provide evidence that several fast sequential conformational changes occur in Fpg after binding to its substrate, converting the protein into a catalytically active conformation.
Figures


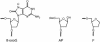
Similar articles
-
Pre-steady-state kinetic study of substrate specificity of Escherichia coli formamidopyrimidine--DNA glycosylase.Biochemistry. 2007 Jan 16;46(2):424-35. doi: 10.1021/bi060787r. Biochemistry. 2007. PMID: 17209553
-
Solution-state NMR investigation of DNA binding interactions in Escherichia coli formamidopyrimidine-DNA glycosylase (Fpg): a dynamic description of the DNA/protein interface.DNA Repair (Amst). 2005 Mar 2;4(3):327-39. doi: 10.1016/j.dnarep.2004.09.012. DNA Repair (Amst). 2005. PMID: 15661656
-
Reversible chemical step and rate-limiting enzyme regeneration in the reaction catalyzed by formamidopyrimidine-DNA glycosylase.Biochemistry. 2009 Dec 8;48(48):11335-43. doi: 10.1021/bi901100b. Biochemistry. 2009. PMID: 19835417
-
Real-time studies of conformational dynamics of the repair enzyme E. coli formamidopyrimidine-DNA glycosylase and its DNA complexes during catalytic cycle.Mutat Res. 2010 Mar 1;685(1-2):3-10. doi: 10.1016/j.mrfmmm.2009.08.018. Epub 2009 Sep 12. Mutat Res. 2010. PMID: 19751748 Review.
-
Recognition of damaged DNA by Escherichia coli Fpg protein: insights from structural and kinetic data.Mutat Res. 2003 Oct 29;531(1-2):141-56. doi: 10.1016/j.mrfmmm.2003.09.002. Mutat Res. 2003. PMID: 14637251 Review.
Cited by
-
Visualizing the Search for Radiation-damaged DNA Bases in Real Time.Radiat Phys Chem Oxf Engl 1993. 2016 Nov;128:126-133. doi: 10.1016/j.radphyschem.2016.05.011. Epub 2016 May 13. Radiat Phys Chem Oxf Engl 1993. 2016. PMID: 27818579 Free PMC article.
-
Modulation of the turnover of formamidopyrimidine DNA glycosylase.Biochemistry. 2006 Jun 13;45(23):7341-6. doi: 10.1021/bi052383p. Biochemistry. 2006. PMID: 16752923 Free PMC article.
-
A dynamic checkpoint in oxidative lesion discrimination by formamidopyrimidine-DNA glycosylase.Nucleic Acids Res. 2016 Jan 29;44(2):683-94. doi: 10.1093/nar/gkv1092. Epub 2015 Nov 8. Nucleic Acids Res. 2016. PMID: 26553802 Free PMC article.
-
Catalytic mechanism of Escherichia coli endonuclease VIII: roles of the intercalation loop and the zinc finger.Biochemistry. 2006 Oct 3;45(39):12039-49. doi: 10.1021/bi060663e. Biochemistry. 2006. PMID: 17002303 Free PMC article.
-
Mutational and Kinetic Analysis of Lesion Recognition by Escherichia coli Endonuclease VIII.Genes (Basel). 2017 May 13;8(5):140. doi: 10.3390/genes8050140. Genes (Basel). 2017. PMID: 28505099 Free PMC article.
References
-
- von Sonntag C. (1987) The Chemical Basis of Radiation Biology. Taylor & Francis, London.
-
- Halliwell B. and Gutteridge,J.M.C. (1999) Free Radicals in Biology and Medicine, 3rd Edn. Oxford University Press, Oxford, UK.
-
- Shibutani S., Takeshita,M. and Grollman,A.P. (1991) Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature, 349, 431–434. - PubMed
-
- Moriya M., Ou,C., Bodepudi,V., Johnson,F., Takeshita,M. and Grollman,A.P. (1991) Site-specific mutagenesis using a gapped duplex vector: a study of translesion synthesis past 8-oxodeoxyguanosine in E. coli. Mutat. Res., 254, 281–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

