Calcineurin regulates cyclin D1 accumulation in growth-stimulated fibroblasts
- PMID: 14767060
- PMCID: PMC379279
- DOI: 10.1091/mbc.e03-10-0730
Calcineurin regulates cyclin D1 accumulation in growth-stimulated fibroblasts
Abstract
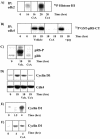
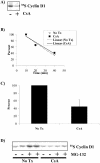
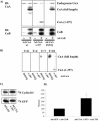
Calcium (Ca(2+)) and calmodulin (CaM) are required for progression of mammalian cells from quiescence into S phase. In multiple cell types, cyclosporin A causes a G(1) cell cycle arrest, implicating the serine/threonine phosphatase calcineurin as one Ca(2+)/CaM-dependent enzyme required for G(1) transit. Here, we show, in diploid human fibroblasts, that cyclosporin A arrested cells in G(1) before cyclin D/cdk4 complex activation and retinoblastoma hyperphosphorylation. This arrest occurred in early G(1) with low levels of cyclin D1 protein. Because cyclin D1 mRNA was induced normally in the cyclosporin A-treated cells, we analyzed the half-life of cyclin D1 in the presence of cyclosporin A and found no difference from control cells. However, cyclosporin A treatment dramatically reduced cyclin D1 protein synthesis. Although these pharmacological experiments suggested that calcineurin regulates cyclin D1 synthesis, we evaluated the effects of overexpression of activated calcineurin on cyclin D1 synthesis. In contrast to the reduction of cyclin D1 with cyclosporin A, ectopic expression of calcium/calmodulin-independent calcineurin promoted synthesis of cyclin D1 during G(1) progression. Therefore, calcineurin is a Ca(2+)/CaM-dependent target that regulates cyclin D1 accumulation in G(1).
Figures


Similar articles
-
CaMK-II inhibition reduces cyclin D1 levels and enhances the association of p27kip1 with Cdk2 to cause G1 arrest in NIH 3T3 cells.Exp Cell Res. 1998 May 1;240(2):218-27. doi: 10.1006/excr.1997.3925. Exp Cell Res. 1998. PMID: 9596994
-
Inhibitors of calcineurin block expression of cyclins A and E induced by fibroblast growth factor in Swiss 3T3 fibroblasts.Arch Biochem Biophys. 1998 May 15;353(2):374-8. doi: 10.1006/abbi.1998.0667. Arch Biochem Biophys. 1998. PMID: 9606972
-
Disruption of the actin cytoskeleton leads to inhibition of mitogen-induced cyclin E expression, Cdk2 phosphorylation, and nuclear accumulation of the retinoblastoma protein-related p107 protein.Exp Cell Res. 2000 Aug 25;259(1):35-53. doi: 10.1006/excr.2000.4966. Exp Cell Res. 2000. PMID: 10942577
-
Cyclin D1 functions in cell migration.Cell Cycle. 2006 Nov 1;5(21):2440-2. doi: 10.4161/cc.5.21.3428. Epub 2006 Sep 22. Cell Cycle. 2006. PMID: 17106256 Review.
-
Decoding the Phosphatase Code: Regulation of Cell Proliferation by Calcineurin.Int J Mol Sci. 2022 Jan 20;23(3):1122. doi: 10.3390/ijms23031122. Int J Mol Sci. 2022. PMID: 35163061 Free PMC article. Review.
Cited by
-
Mitochondrial Ca2+ Signaling in Health, Disease and Therapy.Cells. 2021 May 25;10(6):1317. doi: 10.3390/cells10061317. Cells. 2021. PMID: 34070562 Free PMC article. Review.
-
Calcium wave signaling in cancer cells.Life Sci. 2010 Nov 20;87(19-22):587-95. doi: 10.1016/j.lfs.2010.09.013. Epub 2010 Sep 25. Life Sci. 2010. PMID: 20875431 Free PMC article. Review.
-
Mechanisms Underlying Influence of Bioelectricity in Development.Front Cell Dev Biol. 2022 Feb 14;10:772230. doi: 10.3389/fcell.2022.772230. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35237593 Free PMC article. Review.
-
A temporal examination of calcium signaling in cancer- from tumorigenesis, to immune evasion, and metastasis.Cell Biosci. 2018 Apr 3;8:25. doi: 10.1186/s13578-018-0223-5. eCollection 2018. Cell Biosci. 2018. PMID: 29636894 Free PMC article. Review.
-
Cyclosporine a mediates pathogenesis of aggressive cutaneous squamous cell carcinoma by augmenting epithelial-mesenchymal transition: role of TGFβ signaling pathway.Mol Carcinog. 2011 Jul;50(7):516-27. doi: 10.1002/mc.20744. Epub 2011 Feb 9. Mol Carcinog. 2011. PMID: 21308804 Free PMC article.
References
-
- Aramburu, J., Rao, A., and Klee, C.B. (2000). Calcineurin: from structure to function. Curr. Top. Cell Regul. 36, 237-295. - PubMed
-
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.O., Seidman, T.G., Smith, J.A., and Struhl, K. eds (1999). Short protocols in molecular biology. New York, NY; John Wiley & Sons.
-
- Baksh, S., Widlund, H.R., Frazer-Abel, A.A., Du, J., Fosmire, S., Fisher, D.E., DeCaprio, J.A., Modiano, J.F., and Burakoff, S.J. (2002). NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol. Cell 10, 1071-1081. - PubMed
-
- Boynton, A.L., Whitfield, J.F., Isaacs, R.J., and Tremblay, R. (1977). The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J. Cell. Physiol. 92, 241-247. - PubMed
-
- Brooks-Frederich, K.M., Cianciarulo, F.L., Rittling, S.R., and Cristofalo, V.J. (1993). Cell cycle-dependent regulation of Ca2+ in young and senescent WI-38 cells. Exp. Cell Res. 205, 412-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

