Chemical-genetic inhibition of a sensitized mutant myosin Vb demonstrates a role in peripheral-pericentriolar membrane traffic
- PMID: 14766983
- PMCID: PMC357019
- DOI: 10.1073/pnas.0305895101
Chemical-genetic inhibition of a sensitized mutant myosin Vb demonstrates a role in peripheral-pericentriolar membrane traffic
Abstract
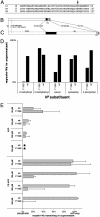
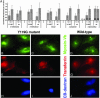
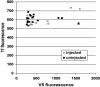
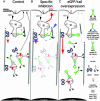
Selective, in situ inhibition of individual unconventional myosins is a powerful approach to determine their specific physiological functions. Here, we report the engineering of a myosin Vb mutant that still hydrolyzes ATP, yet is selectively sensitized to an N(6)-substituted ADP analog that inhibits its activity, causing it to remain tightly bound to actin. Inhibition of the sensitized mutant causes inhibition of accumulation of transferrin in the cytoplasm and increases levels of plasma-membrane transferrin receptor, suggesting that myosin Vb functions in traffic between peripheral and pericentrosomal compartments.
Figures

Similar articles
-
Myosin-Vb functions as a dynamic tether for peripheral endocytic compartments during transferrin trafficking.BMC Cell Biol. 2008 Aug 7;9:44. doi: 10.1186/1471-2121-9-44. BMC Cell Biol. 2008. PMID: 18687135 Free PMC article.
-
Myosin vb is associated with plasma membrane recycling systems.Mol Biol Cell. 2001 Jun;12(6):1843-57. doi: 10.1091/mbc.12.6.1843. Mol Biol Cell. 2001. PMID: 11408590 Free PMC article.
-
Human myosin-Vc is a novel class V myosin expressed in epithelial cells.J Cell Sci. 2002 Mar 1;115(Pt 5):991-1004. doi: 10.1242/jcs.115.5.991. J Cell Sci. 2002. PMID: 11870218
-
Chemical-genetic inhibition of sensitized mutant unconventional myosins.Methods Mol Biol. 2007;392:231-40. doi: 10.1007/978-1-59745-490-2_16. Methods Mol Biol. 2007. PMID: 17951722
-
Holding the reins on myosin V.Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13719-20. doi: 10.1073/pnas.0507068102. Epub 2005 Sep 19. Proc Natl Acad Sci U S A. 2005. PMID: 16172373 Free PMC article. Review. No abstract available.
Cited by
-
The Bump-and-Hole Tactic: Expanding the Scope of Chemical Genetics.Cell Chem Biol. 2018 Oct 18;25(10):1171-1184. doi: 10.1016/j.chembiol.2018.07.001. Epub 2018 Aug 2. Cell Chem Biol. 2018. PMID: 30078633 Free PMC article. Review.
-
Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3.Mol Biol Cell. 2007 Aug;18(8):2828-37. doi: 10.1091/mbc.e07-02-0169. Epub 2007 May 16. Mol Biol Cell. 2007. PMID: 17507647 Free PMC article.
-
Myosin-Vb functions as a dynamic tether for peripheral endocytic compartments during transferrin trafficking.BMC Cell Biol. 2008 Aug 7;9:44. doi: 10.1186/1471-2121-9-44. BMC Cell Biol. 2008. PMID: 18687135 Free PMC article.
-
CART: an Hrs/actinin-4/BERP/myosin V protein complex required for efficient receptor recycling.Mol Biol Cell. 2005 May;16(5):2470-82. doi: 10.1091/mbc.e04-11-1014. Epub 2005 Mar 16. Mol Biol Cell. 2005. PMID: 15772161 Free PMC article.
-
Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol.Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):10775-82. doi: 10.1073/pnas.0912925107. Epub 2010 May 21. Proc Natl Acad Sci U S A. 2010. PMID: 20495089 Free PMC article. Review.
References
-
- Bishop, A. C., Buzko, O. & Shokat, K. M. (2001) Trends Cell Biol. 11, 167–172. - PubMed
-
- Gulick, A. M., Bauer, C. B., Thoden, J. B. & Rayment, I. (1997) Biochemistry 36, 11619–11628. - PubMed
-
- Gillespie, P. G., Gillespie, S. K. H., Mercer, J. A., Shah, K. & Shokat, K. M. (1999) J. Biol. Chem. 274, 31373–31381. - PubMed
-
- Holt, J. R., Gillespie, S. K. H., Provance, D. W., Shah, K., Shokat, K. M., Corey, D. P., Mercer, J. A. & Gillespie, P. G. (2002) Cell 108, 371–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

