Ca2+ syntillas, miniature Ca2+ release events in terminals of hypothalamic neurons, are increased in frequency by depolarization in the absence of Ca2+ influx
- PMID: 14762141
- PMCID: PMC6793580
- DOI: 10.1523/JNEUROSCI.4286-03.2004
Ca2+ syntillas, miniature Ca2+ release events in terminals of hypothalamic neurons, are increased in frequency by depolarization in the absence of Ca2+ influx
Abstract
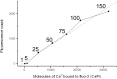
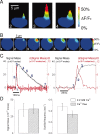
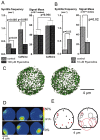
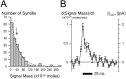
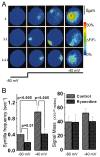
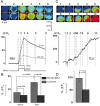
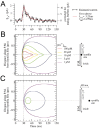
Localized, brief Ca2+ transients (Ca2+ syntillas) caused by release from intracellular stores were found in isolated nerve terminals from magnocellular hypothalamic neurons and examined quantitatively using a signal mass approach to Ca2+ imaging. Ca2+ syntillas (scintilla, L., spark, from a synaptic structure, a nerve terminal) are caused by release of approximately 250,000 Ca ions on average by a Ca2+ flux lasting on the order of tens of milliseconds and occur spontaneously at a membrane potential of -80 mV. Syntillas are unaffected by removal of extracellular Ca2+, are mediated by ryanodine receptors (RyRs) and are increased in frequency, in the absence of extracellular Ca2+, by physiological levels of depolarization. This represents the first direct demonstration of mobilization of Ca2+ from intracellular stores in neurons by depolarization without Ca2+ influx. The regulation of syntillas by depolarization provides a new link between neuronal activity and cytosolic [Ca2+] in nerve terminals.
Figures
Similar articles
-
Syntillas release Ca2+ at a site different from the microdomain where exocytosis occurs in mouse chromaffin cells.Biophys J. 2006 Mar 15;90(6):2027-37. doi: 10.1529/biophysj.105.071654. Epub 2005 Dec 30. Biophys J. 2006. PMID: 16387759 Free PMC article.
-
Suppression of Ca2+ syntillas increases spontaneous exocytosis in mouse adrenal chromaffin cells.J Gen Physiol. 2009 Oct;134(4):267-80. doi: 10.1085/jgp.200910285. J Gen Physiol. 2009. PMID: 19786582 Free PMC article.
-
Type 1 ryanodine receptor knock-in mutation causing central core disease of skeletal muscle also displays a neuronal phenotype.Proc Natl Acad Sci U S A. 2012 Jan 10;109(2):610-5. doi: 10.1073/pnas.1115111108. Epub 2011 Dec 27. Proc Natl Acad Sci U S A. 2012. PMID: 22203976 Free PMC article.
-
Interplay between ER Ca2+ uptake and release fluxes in neurons and its impact on [Ca2+] dynamics.Biol Res. 2004;37(4):665-74. doi: 10.4067/s0716-97602004000400024. Biol Res. 2004. PMID: 15709696 Review.
-
Calcium-induced calcium release in skeletal muscle.Physiol Rev. 2009 Oct;89(4):1153-76. doi: 10.1152/physrev.00040.2008. Physiol Rev. 2009. PMID: 19789379 Review.
Cited by
-
Dihydropyridine receptors and type 1 ryanodine receptors constitute the molecular machinery for voltage-induced Ca2+ release in nerve terminals.J Neurosci. 2006 Jul 19;26(29):7565-74. doi: 10.1523/JNEUROSCI.1512-06.2006. J Neurosci. 2006. PMID: 16855084 Free PMC article.
-
Homeostatic and stimulus-induced coupling of the L-type Ca2+ channel to the ryanodine receptor in the hippocampal neuron in slices.Cell Calcium. 2009 Jul;46(1):30-8. doi: 10.1016/j.ceca.2009.03.018. Epub 2009 May 2. Cell Calcium. 2009. PMID: 19411104 Free PMC article.
-
Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex.Proc Natl Acad Sci U S A. 2007 May 1;104(18):7646-51. doi: 10.1073/pnas.0701944104. Epub 2007 Apr 25. Proc Natl Acad Sci U S A. 2007. PMID: 17460041 Free PMC article.
-
Modulation/physiology of calcium channel sub-types in neurosecretory terminals.Cell Calcium. 2012 Mar-Apr;51(3-4):284-92. doi: 10.1016/j.ceca.2012.01.008. Epub 2012 Feb 17. Cell Calcium. 2012. PMID: 22341671 Free PMC article. Review.
-
Calcium Contributes to Polarized Targeting of HIV Assembly Machinery by Regulating Complex Stability.JACS Au. 2022 Feb 1;2(2):522-530. doi: 10.1021/jacsau.1c00563. eCollection 2022 Feb 28. JACS Au. 2022. PMID: 35253001 Free PMC article.
References
-
- Becker PL, Singer JJ, Walsh JVJ, Fay FS (1989) Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science 244: 211–214. - PubMed
-
- Berridge MJ (1998) Neuronal calcium signaling. Neuron 21: 13–26. - PubMed
-
- Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415: 198–205. - PubMed
-
- Betz WJ, Angleson JK (1998) The synaptic vesicle cycle. Annu Rev Physiol 60: 347–363. - PubMed
-
- Burbach JP, Luckman SM, Murphy D, Gainer H (2001) Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev 81: 1197–1267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
