Discrete domains within the rotavirus VP5* direct peripheral membrane association and membrane permeability
- PMID: 14747568
- PMCID: PMC369428
- DOI: 10.1128/jvi.78.4.2037-2044.2004
Discrete domains within the rotavirus VP5* direct peripheral membrane association and membrane permeability
Abstract
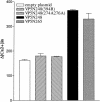
Cleavage of the rotavirus spike protein, VP4, is required for rotavirus-induced membrane permeability and viral entry into cells. The VP5* cleavage product selectively permeabilizes membranes and liposomes and contains an internal hydrophobic domain that is required for membrane permeability. Here we investigate VP5* domains (residues 248 to 474) that direct membrane binding. We determined that expressed VP5 fragments containing residues 248 to 474 or 265 to 474, including the internal hydrophobic domain, bind to cellular membranes but are not present in Triton X-100-resistant membrane rafts. Expressed VP5 partitions into aqueous but not detergent phases of Triton X-114, suggesting that VP5 is not integrally inserted into membranes. Since high-salt or alkaline conditions eluted VP5 from membranes, our findings demonstrate that VP5 is peripherally associated with membranes. Interestingly, mutagenesis of residue 394 (W-->R) within the VP5 hydrophobic domain, which abolishes VP5-directed permeability, had no effect on VP5's peripheral membrane association. In contrast, deletion of N-terminal VP5 residues (residues 265 to 279) abolished VP5 binding to membranes. Alanine mutagenesis of two positively charged residues within this domain (residues 274R and 276K) dramatically reduced (>95%) binding of VP5 to membranes and suggested their potential interaction with polar head groups of the lipid bilayer. Mutations in either the VP5 hydrophobic or basic domain blocked VP5-directed permeability of cells. These findings indicate that there are at least two discrete domains within VP5* required for pore formation: an N-terminal basic domain that permits VP5* to peripherally associate with membranes and an internal hydrophobic domain that is essential for altering membrane permeability. These results provide a fundamental understanding of interactions between VP5* and the membrane, which are required for rotavirus entry.
Figures

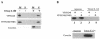
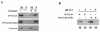

Similar articles
-
Selective membrane permeabilization by the rotavirus VP5* protein is abrogated by mutations in an internal hydrophobic domain.J Virol. 2000 Jul;74(14):6368-76. doi: 10.1128/jvi.74.14.6368-6376.2000. J Virol. 2000. PMID: 10864647 Free PMC article.
-
Effect of mutations in VP5 hydrophobic loops on rotavirus cell entry.J Virol. 2010 Jun;84(12):6200-7. doi: 10.1128/JVI.02461-09. Epub 2010 Apr 7. J Virol. 2010. PMID: 20375171 Free PMC article.
-
Rotavirus capsid protein VP5* permeabilizes membranes.J Virol. 1999 Apr;73(4):3147-53. doi: 10.1128/JVI.73.4.3147-3153.1999. J Virol. 1999. PMID: 10074166 Free PMC article.
-
A rotavirus spike protein conformational intermediate binds lipid bilayers.J Virol. 2010 Feb;84(4):1764-70. doi: 10.1128/JVI.01682-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007281 Free PMC article.
-
Rotavirus protein expression is important for virus assembly and pathogenesis.Arch Virol Suppl. 1996;12:69-77. doi: 10.1007/978-3-7091-6553-9_8. Arch Virol Suppl. 1996. PMID: 9015103 Review.
Cited by
-
An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature.Virology. 2010 Jun 20;402(1):11-9. doi: 10.1016/j.virol.2010.03.043. Epub 2010 Apr 20. Virology. 2010. PMID: 20409568 Free PMC article.
-
Rotavirus assembly: an alternative model that utilizes an atypical trafficking pathway.Curr Top Microbiol Immunol. 2006;309:245-61. doi: 10.1007/3-540-30773-7_9. Curr Top Microbiol Immunol. 2006. PMID: 16909902 Free PMC article. Review.
-
Characterization of neuraminidase-resistant mutants derived from rotavirus porcine strain OSU.J Virol. 2005 Aug;79(16):10369-75. doi: 10.1128/JVI.79.16.10369-10375.2005. J Virol. 2005. PMID: 16051829 Free PMC article.
-
A chaperone-activated nonenveloped virus perforates the physiologically relevant endoplasmic reticulum membrane.J Virol. 2007 Dec;81(23):12996-3004. doi: 10.1128/JVI.01037-07. Epub 2007 Sep 19. J Virol. 2007. PMID: 17881435 Free PMC article.
-
Cytopathic mechanisms of HIV-1.Virol J. 2007 Oct 18;4:100. doi: 10.1186/1743-422X-4-100. Virol J. 2007. PMID: 17945027 Free PMC article. Review.
References
-
- Arias, C. F., P. Isa, C. A. Guerrero, E. Mendez, S. Zarate, T. Lopez, R. Espinosa, P. Romero, and S. Lopez. 2002. Molecular biology of rotavirus cell entry. Arch. Med. Res. 33:356-361. - PubMed
-
- Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. - PubMed
-
- Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

