Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation
- PMID: 14744997
- PMCID: PMC2172232
- DOI: 10.1083/jcb.200304135
Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation
Abstract
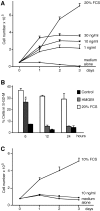
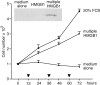
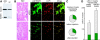
High mobility group box 1 (HMGB1) is an abundant chromatin protein that acts as a cytokine when released in the extracellular milieu by necrotic and inflammatory cells. Here, we show that extracellular HMGB1 and its receptor for advanced glycation end products (RAGE) induce both migration and proliferation of vessel-associated stem cells (mesoangioblasts), and thus may play a role in muscle tissue regeneration. In vitro, HMGB1 induces migration and proliferation of both adult and embryonic mesoangioblasts, and disrupts the barrier function of endothelial monolayers. In living mice, mesoangioblasts injected into the femoral artery migrate close to HMGB1-loaded heparin-Sepharose beads implanted in healthy muscle, but are unresponsive to control beads. Interestingly, alpha-sarcoglycan null dystrophic muscle contains elevated levels of HMGB1; however, mesoangioblasts migrate into dystrophic muscle even if their RAGE receptor is disabled. This implies that the HMGB1-RAGE interaction is sufficient, but not necessary, for mesoangioblast homing; a different pathway might coexist. Although the role of endogenous HMGB1 in the reconstruction of dystrophic muscle remains to be clarified, injected HMGB1 may be used to promote tissue regeneration.
Copyright The Rockefeller University Press
Figures

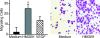


Similar articles
-
Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts.Science. 2003 Jul 25;301(5632):487-92. doi: 10.1126/science.1082254. Epub 2003 Jul 10. Science. 2003. PMID: 12855815
-
HMGB1 and TLR4 mediate skeletal muscle recovery in a murine model of hindlimb ischemia.J Vasc Surg. 2013 Aug;58(2):460-9. doi: 10.1016/j.jvs.2012.11.071. Epub 2013 Feb 12. J Vasc Surg. 2013. PMID: 23414695 Free PMC article.
-
High-mobility group box 1 inhibits gastric ulcer healing through Toll-like receptor 4 and receptor for advanced glycation end products.PLoS One. 2013 Nov 11;8(11):e80130. doi: 10.1371/journal.pone.0080130. eCollection 2013. PLoS One. 2013. PMID: 24244627 Free PMC article.
-
High mobility group box 1 protein, a cue for stem cell recruitment.Biochem Pharmacol. 2004 Sep 15;68(6):1165-70. doi: 10.1016/j.bcp.2004.03.048. Biochem Pharmacol. 2004. PMID: 15313414 Review.
-
Clinical Implications of High-mobility Group Box-1 (HMGB1) and the Receptor for Advanced Glycation End-products (RAGE) in Cutaneous Malignancy: A Systematic Review.Anticancer Res. 2017 Jan;37(1):1-7. doi: 10.21873/anticanres.11282. Anticancer Res. 2017. PMID: 28011467 Review.
Cited by
-
Myocardial regeneration of the failing heart.Heart Fail Rev. 2013 Nov;18(6):815-33. doi: 10.1007/s10741-012-9348-5. Heart Fail Rev. 2013. PMID: 23001638 Review.
-
PAMPs and DAMPs as triggers for DIC.J Intensive Care. 2014 Dec 31;2(1):67. doi: 10.1186/s40560-014-0065-0. eCollection 2014. J Intensive Care. 2014. PMID: 25705424 Free PMC article. Review.
-
TLR4 Deters Perfusion Recovery and Upregulates Toll-like Receptor 2 (TLR2) in Ischemic Skeletal Muscle and Endothelial Cells.Mol Med. 2015 Jul 14;21(1):605-15. doi: 10.2119/molmed.2014.00260. Mol Med. 2015. PMID: 26181630 Free PMC article.
-
High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism.Diabetes. 2010 Jun;59(6):1496-505. doi: 10.2337/db09-1507. Epub 2010 Mar 3. Diabetes. 2010. PMID: 20200317 Free PMC article.
-
Glycyrrhizin inhibits interleukin-8 production and nuclear factor-kappaB activity in lung epithelial cells, but not through glucocorticoid receptors.J Pharmacol Sci. 2008 Mar;106(3):460-8. doi: 10.1254/jphs.fp0072378. Epub 2008 Mar 12. J Pharmacol Sci. 2008. PMID: 18344608 Free PMC article.
References
-
- Abraham, E., J. Arcaroli, A. Carmody, H. Wang, and K.J. Tracey. 2000. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 165:2950–2954. - PubMed
-
- Agresti, A., and M.E. Bianchi. 2003. HMGB proteins and gene expression. Curr. Opin. Genet. Dev. 13:170–178. - PubMed
-
- Bianchi, M.E., and A. Manfredi. 2004. Chromatin and cell death. Biochim. Biophys. Acta. In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

