Thermal stability of human alpha-crystallins sensed by amide hydrogen exchange
- PMID: 14739319
- PMCID: PMC2286712
- DOI: 10.1110/ps.03180004
Thermal stability of human alpha-crystallins sensed by amide hydrogen exchange
Abstract
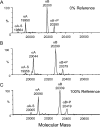
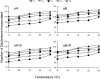
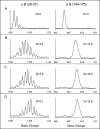
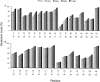
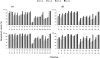
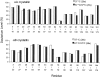
The alpha-crystallins, alphaA and alphaB, are major lens structural proteins with chaperone-like activity and sequence homology to small heat-shock proteins. As yet, their crystal structures have not been determined because of the large size and heterogeneity of the assemblies they form in solution. Because alpha-crystallin chaperone activity increases with temperature, understanding structural changes of alpha-crystallin as it is heated may help elucidate the mechanism of chaperone activity. Although a variety of techniques have been used to probe changes in heat-stressed alpha-crystallin, the results have not yet yielded a clear understanding of chaperone activity. We report examination of native assemblies of human lens alpha-crystallin using hydrogen/deuterium exchange in conjunction with enzymatic digestion and analysis by mass spectrometry. This technique has the advantage of sensing structural changes along much of the protein backbone and being able to detect changes specific to alphaA and alphaB in the native assembly. The reactivity of the amide linkages to hydrogen/deuterium exchange was determined for 92% of the sequence of alphaA and 99% of alphaB. The behavior of alphaA and alphaB is remarkably similar. At low temperatures, there are regions at the beginning of the alpha-crystallin domains in both alphaA and alphaB that have high protection to isotope exchange, whereas the C termini offer little protection. The N terminus of alphaA also has low protection. With increasing temperatures, both proteins show gradual unfolding. The maximum percent change in exposure with increasing temperatures was found in alphaA 72-75 and alphaB 76-79, two regions considered critical for chaperone activity.
Figures

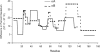
Similar articles
-
Alpha-crystallin regions affected by adenosine 5'-triphosphate identified by hydrogen-deuterium exchange.Biochemistry. 2002 Dec 31;41(52):15876-82. doi: 10.1021/bi026568x. Biochemistry. 2002. PMID: 12501218
-
Probing alpha-crystallin structure using chemical cross-linkers and mass spectrometry.Mol Vis. 2004 Nov 16;10:857-66. Mol Vis. 2004. PMID: 15570221
-
Role of the C-terminal extensions of alpha-crystallins. Swapping the C-terminal extension of alpha-crystallin to alphaB-crystallin results in enhanced chaperone activity.J Biol Chem. 2002 Nov 29;277(48):45821-8. doi: 10.1074/jbc.M206499200. Epub 2002 Sep 15. J Biol Chem. 2002. PMID: 12235146
-
Structure and function of α-crystallins: Traversing from in vitro to in vivo.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):149-66. doi: 10.1016/j.bbagen.2015.06.008. Epub 2015 Jun 25. Biochim Biophys Acta. 2016. PMID: 26116912 Review.
-
Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):258-68. doi: 10.1016/j.bbagen.2015.05.016. Epub 2015 May 27. Biochim Biophys Acta. 2016. PMID: 26026469 Free PMC article. Review.
Cited by
-
Mass Spectrometry Methods for Measuring Protein Stability.Chem Rev. 2022 Apr 27;122(8):7690-7719. doi: 10.1021/acs.chemrev.1c00857. Epub 2022 Mar 22. Chem Rev. 2022. PMID: 35316030 Free PMC article. Review.
-
The structure and oxidation of the eye lens chaperone αA-crystallin.Nat Struct Mol Biol. 2019 Dec;26(12):1141-1150. doi: 10.1038/s41594-019-0332-9. Epub 2019 Dec 2. Nat Struct Mol Biol. 2019. PMID: 31792453 Free PMC article.
-
Changes in solvent accessibility of wild-type and deamidated βB2-crystallin following complex formation with αA-crystallin.Exp Eye Res. 2012 Nov;104:48-58. doi: 10.1016/j.exer.2012.09.001. Epub 2012 Sep 12. Exp Eye Res. 2012. PMID: 22982024 Free PMC article.
-
The reaction of alpha-crystallin with the cross-linker 3,3'-dithiobis(sulfosuccinimidyl propionate) demonstrates close proximity of the C termini of alphaA and alphaB in the native assembly.Protein Sci. 2004 Oct;13(10):2832-5. doi: 10.1110/ps.04910004. Protein Sci. 2004. PMID: 15388868 Free PMC article.
References
-
- Abgar, S., Backman, J., Aerts, T., Vanhoudt, J., and Clauwaert, J. 2000. The structural differences between bovine lens αA- and αB-crystallin. Eur. J. Biochem. 267 5916–5925. - PubMed
-
- ———. 1994. Protein stability parameters measured by hydrogen exchange. Proteins 20 4–14. - PubMed
-
- Berengian, A.R., Bova, M.P., and Mchaourab, H.S. 1997. Structure and function of the conserved domain in αA-crystallin. Site-directed spin labeling identifies a β-strand located near a subunit interface. Biochemistry 36 9951–9957. - PubMed
-
- Berghuis, A.M. and Brayer, G.D. 1992. Oxidation state-dependent conformational changes in cytochrome c. J. Mol. Biol. 223 959–976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

