Persistent progenitors at the retinal margin of ptc+/- mice
- PMID: 14715955
- PMCID: PMC6729562
- DOI: 10.1523/JNEUROSCI.2980-03.2004
Persistent progenitors at the retinal margin of ptc+/- mice
Abstract
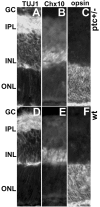
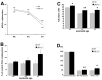
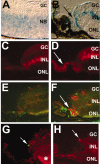
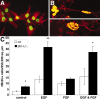
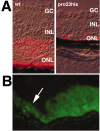
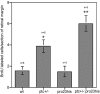
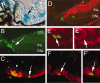
The hedgehog signaling pathway is a key regulator of neural development, affecting both proliferation and differentiation of neural progenitors. Sonic hedgehog (Shh) is a mitogenic factor for retinal progenitors in vitro. To determine whether this signaling system is important in vivo for regulating retinal progenitor proliferation, we analyzed mice with a single functional allele of the Shh receptor patched (ptc). We found that ptc+/- mice had increased numbers of neural progenitors at every stage of retinal development that we examined. In addition, these mice had persistent progenitors at the retinal margin for up to 3 months of age, reminiscent of the ciliary marginal zone of lower vertebrates. To test whether the progenitors at the retinal margin of ptc+/- mice could be induced to regenerate retinal neurons in response to damage, we bred ptc+/- mice onto a retinal degeneration background (pro23his rhodopsin transgenic) and labeled newly generated cells with combined immunohistochemistry for bromodeoxyuridine and retinal neuron and photoreceptor-specific markers. We found newly generated neurons and photoreceptors at the retinal margin in ptc+/-;pro23his mice. We propose that the Shh pathway may act as a regulator of both prenatal and postnatal retinal growth.
Figures

Similar articles
-
Differential gene induction by genetic and ligand-mediated activation of the Sonic hedgehog pathway in neural stem cells.Dev Biol. 2007 Aug 15;308(2):331-42. doi: 10.1016/j.ydbio.2007.05.031. Epub 2007 May 31. Dev Biol. 2007. PMID: 17599824
-
Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens.Dev Biol. 2000 Apr 15;220(2):197-210. doi: 10.1006/dbio.2000.9640. Dev Biol. 2000. PMID: 10753510
-
Medulloblastoma tumorigenesis diverges from cerebellar granule cell differentiation in patched heterozygous mice.Dev Biol. 2003 Nov 1;263(1):50-66. doi: 10.1016/s0012-1606(03)00434-2. Dev Biol. 2003. PMID: 14568546
-
The role of the hedgehog/patched signaling pathway in epithelial stem cell proliferation: from fly to human.Cell Res. 1998 Mar;8(1):15-21. doi: 10.1038/cr.1998.2. Cell Res. 1998. PMID: 9570013 Review.
-
Neural regeneration in the chick retina.Prog Retin Eye Res. 2005 Mar;24(2):161-82. doi: 10.1016/j.preteyeres.2004.07.003. Epub 2004 Nov 11. Prog Retin Eye Res. 2005. PMID: 15610972 Review.
Cited by
-
Intravitreal injection of ciliary neurotrophic factor (CNTF) causes peripheral remodeling and does not prevent photoreceptor loss in canine RPGR mutant retina.Exp Eye Res. 2007 Apr;84(4):753-71. doi: 10.1016/j.exer.2006.12.019. Epub 2007 Jan 9. Exp Eye Res. 2007. PMID: 17320077 Free PMC article.
-
Neural regeneration and cell replacement: a view from the eye.Cell Stem Cell. 2008 Jun 5;2(6):538-49. doi: 10.1016/j.stem.2008.05.002. Cell Stem Cell. 2008. PMID: 18522847 Free PMC article. Review.
-
Distinct neurogenic potential in the retinal margin and the pars plana of mammalian eye.J Neurosci. 2012 Sep 12;32(37):12797-807. doi: 10.1523/JNEUROSCI.0118-12.2012. J Neurosci. 2012. PMID: 22973003 Free PMC article.
-
Identification of a stem cell niche in the zone of Ranvier within the knee joint.J Anat. 2009 Sep;215(3):355-63. doi: 10.1111/j.1469-7580.2009.01115.x. Epub 2009 Jun 26. J Anat. 2009. PMID: 19563472 Free PMC article.
-
Near complete loss of retinal ganglion cells in the math5/brn3b double knockout elicits severe reductions of other cell types during retinal development.Dev Biol. 2008 Apr 15;316(2):214-27. doi: 10.1016/j.ydbio.2008.01.015. Epub 2008 Jan 26. Dev Biol. 2008. PMID: 18321480 Free PMC article.
References
-
- Ahmad I, Tang L, Pham H (2000) Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun 270: 517-521. - PubMed
-
- Alvarez-Buylla A, Garcia-Verdago JM, Tramontih AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 4: 287-293. - PubMed
-
- Britto J, Tannahill D, Keynes R (2002) A critical role for sonic hedgehog signaling in the early expansion of the developing brain. Nat Neurosci 5: 103-110. - PubMed
-
- Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M (2002) Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone. Eur J Neurosci 16: 2351-2357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
