Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder
- PMID: 14699048
- PMCID: PMC327193
- DOI: 10.1073/pnas.0307203101
Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder
Abstract
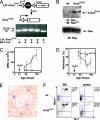
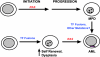
RAS mutations are common in myeloid malignancies; however, it is not known whether oncogenic RAS can initiate leukemia. We show that expressing mutant K-Ras(G12D) protein from the endogenous murine locus rapidly induces a fatal myeloproliferative disorder with 100% penetrance characterized by tissue infiltration, hypersensitivity to growth factors, and hyperproliferation. Hematopoietic cells from diseased mice demonstrated increased levels of Ras-GTP, but effector kinases were not constitutively phosphorylated and responded normally to growth factors. Oncogenic RAS is sufficient to initiate myeloid leukemogenesis in mice, and this provides an in vivo system for biologic and preclinical studies.
Figures

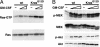

Similar articles
-
Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease.J Clin Invest. 2004 Feb;113(4):528-38. doi: 10.1172/JCI20476. J Clin Invest. 2004. PMID: 14966562 Free PMC article.
-
Hematopoiesis and leukemogenesis in mice expressing oncogenic NrasG12D from the endogenous locus.Blood. 2011 Feb 10;117(6):2022-32. doi: 10.1182/blood-2010-04-280750. Epub 2010 Dec 16. Blood. 2011. PMID: 21163920 Free PMC article.
-
Loss of wild-type Kras promotes activation of all Ras isoforms in oncogenic Kras-induced leukemogenesis.Leukemia. 2016 Jul;30(7):1542-51. doi: 10.1038/leu.2016.40. Epub 2016 Feb 29. Leukemia. 2016. PMID: 27055865 Free PMC article.
-
NOX2 inhibition reduces oxidative stress and prolongs survival in murine KRAS-induced myeloproliferative disease.Oncogene. 2019 Feb;38(9):1534-1543. doi: 10.1038/s41388-018-0528-1. Epub 2018 Oct 15. Oncogene. 2019. PMID: 30323311 Free PMC article.
-
Loss of Dnmt3a and endogenous Kras(G12D/+) cooperate to regulate hematopoietic stem and progenitor cell functions in leukemogenesis.Leukemia. 2015 Sep;29(9):1847-56. doi: 10.1038/leu.2015.85. Epub 2015 Mar 24. Leukemia. 2015. PMID: 25801914 Free PMC article.
Cited by
-
Cooperative loss of RAS feedback regulation drives myeloid leukemogenesis.Nat Genet. 2015 May;47(5):539-43. doi: 10.1038/ng.3251. Epub 2015 Mar 30. Nat Genet. 2015. PMID: 25822087 Free PMC article.
-
Constitutive MAP kinase activation in hematopoietic stem cells induces a myeloproliferative disorder.PLoS One. 2011;6(12):e28350. doi: 10.1371/journal.pone.0028350. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164275 Free PMC article.
-
Effect of Ras inhibition in hematopoiesis and BCR/ABL leukemogenesis.J Hematol Oncol. 2008 Jun 5;1:5. doi: 10.1186/1756-8722-1-5. J Hematol Oncol. 2008. PMID: 18577264 Free PMC article.
-
Cell-intrinsic depletion of Aml1-ETO-expressing pre-leukemic hematopoietic stem cells by K-Ras activating mutation.Haematologica. 2019 Nov;104(11):2215-2224. doi: 10.3324/haematol.2018.205351. Epub 2019 Apr 11. Haematologica. 2019. PMID: 30975913 Free PMC article.
-
Endogenous oncogenic Nras mutation initiates hematopoietic malignancies in a dose- and cell type-dependent manner.Blood. 2011 Jul 14;118(2):368-79. doi: 10.1182/blood-2010-12-326058. Epub 2011 May 17. Blood. 2011. PMID: 21586752 Free PMC article.
References
-
- Bos, J. L. (1989) Cancer Res. 49, 4682–4689. - PubMed
-
- Bourne, H. R., Sanders, D. A. & McCormick, F. (1990) Nature 348, 125–132. - PubMed
-
- Bourne, H. R., Sanders, D. A. & McCormick, F. (1991) Nature 349, 117–127. - PubMed
-
- Boguski, M. & McCormick, F. (1993) Nature 366, 643–653. - PubMed
-
- Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. (1997) Cell 88, 593–602. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

