Incorporation of pol into human immunodeficiency virus type 1 Gag virus-like particles occurs independently of the upstream Gag domain in Gag-pol
- PMID: 14694138
- PMCID: PMC368740
- DOI: 10.1128/jvi.78.2.1042-1049.2004
Incorporation of pol into human immunodeficiency virus type 1 Gag virus-like particles occurs independently of the upstream Gag domain in Gag-pol
Abstract
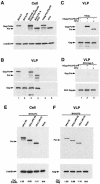
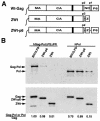
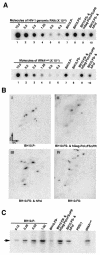
By using particle-associated reverse transcriptase (RT) activity as an assay for Pol incorporation into human immunodeficiency virus type 1 (HIV-1) Gag virus-like particles (VLPs), it has been found that truncated, protease-negative, Gag-Pol missing cis Gag sequences is still incorporated into Gag VLPs, albeit at significantly reduced levels (10 to 20% of the level of wild-type Gag-Pol). In this work, we have directly measured the incorporation of truncated Gag-Pol species into Gag VLPs and have found that truncated Gag-Pol that is missing all sequences upstream of RT is still incorporated into Gag VLPs at levels approximating 70% of that achieved by wild-type Gag-Pol. Neither protease nor integrase regions in Pol are required for its incorporation, implying an interaction between Gag and RT sequences in the Pol protein. While the incorporation of Gag-Pol into Gag VLPs is reduced 12-fold by the replacement of the nucleocapsid within Gag with a leucine zipper motif, this mutation does not affect Pol incorporation. However, the deletion of p6 in Gag reduces Pol incorporation into Gag VLPs four- to fivefold. Pol shows the same ability as Gag-Pol to selectively package tRNA(Lys) into Gag VLPs, and primer tRNA(3)(Lys) is found annealed to the viral genomic RNA. These data suggest that after the initial separation of Gag from Pol during cleavage of Gag-Pol by viral protease, the Pol species still retains the capacity to bind to both Gag and tRNA(3)(Lys), which may be required for Pol and tRNA(3)(Lys) to be retained in the assembling virion until budding is completed.
Figures
Similar articles
-
Incorporation of human immunodeficiency virus type 1 reverse transcriptase into virus-like particles.J Virol. 2007 May;81(10):5155-65. doi: 10.1128/JVI.01796-06. Epub 2007 Mar 7. J Virol. 2007. PMID: 17344306 Free PMC article.
-
Role of Pr160gag-pol in mediating the selective incorporation of tRNA(Lys) into human immunodeficiency virus type 1 particles.J Virol. 1994 Apr;68(4):2065-72. doi: 10.1128/JVI.68.4.2065-2072.1994. J Virol. 1994. PMID: 7511167 Free PMC article.
-
Coding sequences upstream of the human immunodeficiency virus type 1 reverse transcriptase domain in Gag-Pol are not essential for incorporation of the Pr160(gag-pol) into virus particles.J Virol. 2002 Apr;76(7):3221-31. doi: 10.1128/jvi.76.7.3221-3231.2002. J Virol. 2002. PMID: 11884546 Free PMC article.
-
The tRNALys packaging complex in HIV-1.Int J Biochem Cell Biol. 2004 Sep;36(9):1776-86. doi: 10.1016/j.biocel.2004.02.022. Int J Biochem Cell Biol. 2004. PMID: 15183344 Review.
-
Proteolytic processing of foamy virus Gag and Pol proteins.Curr Top Microbiol Immunol. 2003;277:63-88. doi: 10.1007/978-3-642-55701-9_3. Curr Top Microbiol Immunol. 2003. PMID: 12908768 Review.
Cited by
-
Sequential deletion of the integrase (Gag-Pol) carboxyl terminus reveals distinct phenotypic classes of defective HIV-1.J Virol. 2011 May;85(10):4654-66. doi: 10.1128/JVI.02374-10. Epub 2011 Mar 2. J Virol. 2011. PMID: 21367893 Free PMC article.
-
Interferon-Regulated Expression of Cellular Splicing Factors Modulates Multiple Levels of HIV-1 Gene Expression and Replication.Viruses. 2024 Jun 11;16(6):938. doi: 10.3390/v16060938. Viruses. 2024. PMID: 38932230 Free PMC article. Review.
-
The connection domain in reverse transcriptase facilitates the in vivo annealing of tRNALys3 to HIV-1 genomic RNA.Retrovirology. 2004 Oct 19;1:33. doi: 10.1186/1742-4690-1-33. Retrovirology. 2004. PMID: 15494076 Free PMC article.
-
HIV-1 Mutant Assembly, Processing and Infectivity Expresses Pol Independent of Gag.Viruses. 2020 Jan 2;12(1):54. doi: 10.3390/v12010054. Viruses. 2020. PMID: 31906562 Free PMC article.
-
Programmed ribosomal frameshifting in HIV-1 and the SARS-CoV.Virus Res. 2006 Jul;119(1):29-42. doi: 10.1016/j.virusres.2005.10.008. Epub 2005 Nov 28. Virus Res. 2006. PMID: 16310880 Free PMC article. Review.
References
-
- Berkowitz, R., J. Fisher, and S. P. Goff. 1996. RNA packaging, p. 177-218. In H. G. Krausslich (ed.), Morphogenesis and maturation of retroviruses, vol. 214. Springer-Verlag, New York, N.Y.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

